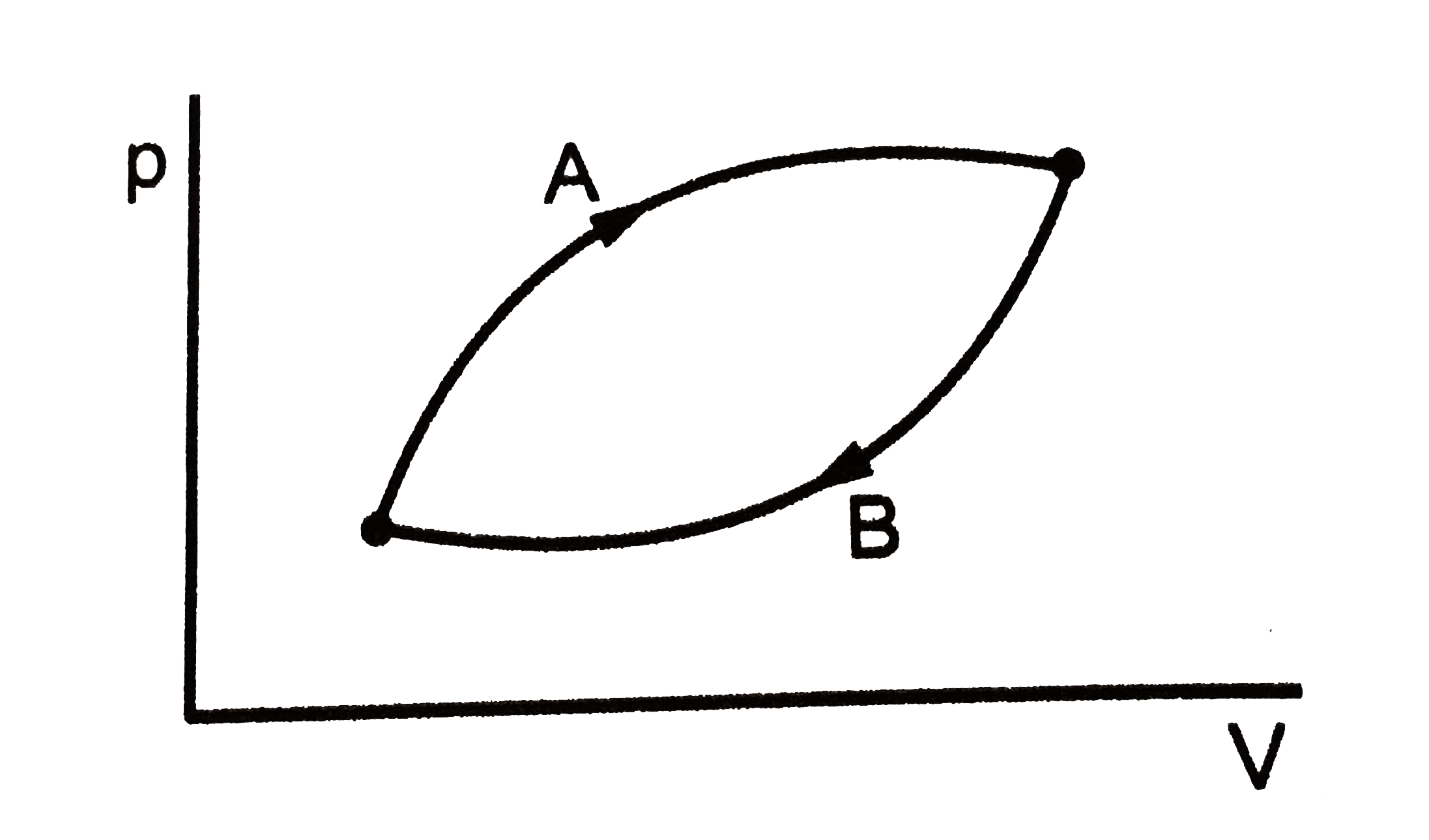
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HC VERMA ENGLISH-LAWS OF THERMODYNAMICS-All Questions
- In a process on a system, the initial pressure and volume are equal to...
Text Solution
|
- A system can be taken from the initial state p(1),V(1) to the final st...
Text Solution
|
- Refer to figure. Let DeltaU(1) and DeltaU(2) be the changes in interna...
Text Solution
|
- The internal enegy of an ideal gas decreases by the same amount as the...
Text Solution
|
- A thermally insulated, closed copper vessel contains water at 15^(0)C....
Text Solution
|
- Shows a peddle wheel couple to a mass of 12 kg through fixed frictionl...
Text Solution
|
- A 100 kg block is started with a speed of 2.0 m s^(-1) on a long, roug...
Text Solution
|
- calculate the change in internal energy of a gas kept in a rigid conta...
Text Solution
|
- the pressure of a gas change linearly with volume from 10kPa, 200 cc t...
Text Solution
|
- An ideal gas is taken from an initial state i to a final state f in s...
Text Solution
|
- shows three paths through which a gas can be taken from the state A to...
Text Solution
|
- when a system is taken through the process abc shown in the fig. 80 J ...
Text Solution
|
- 50 cal of heat should be supplied to take a system from the state A t...
Text Solution
|
- calculate the heat absorbed by a system in going through the cyclic pr...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A substance is taken through the process abc as shown in fig, if the ...
Text Solution
|
- A gas is taken along the path AB as shown in fig, if 70 cal of heat is...
Text Solution
|
- The internal energy of a gas is given by U=1.5pV. It expands from 100...
Text Solution
|
- A gas is enclosed in a cylindrical vessel fitted with a frictionless p...
Text Solution
|
- A gas is initially at a pressure of 100 kPa and its volume is 2.0 m^(3...
Text Solution
|