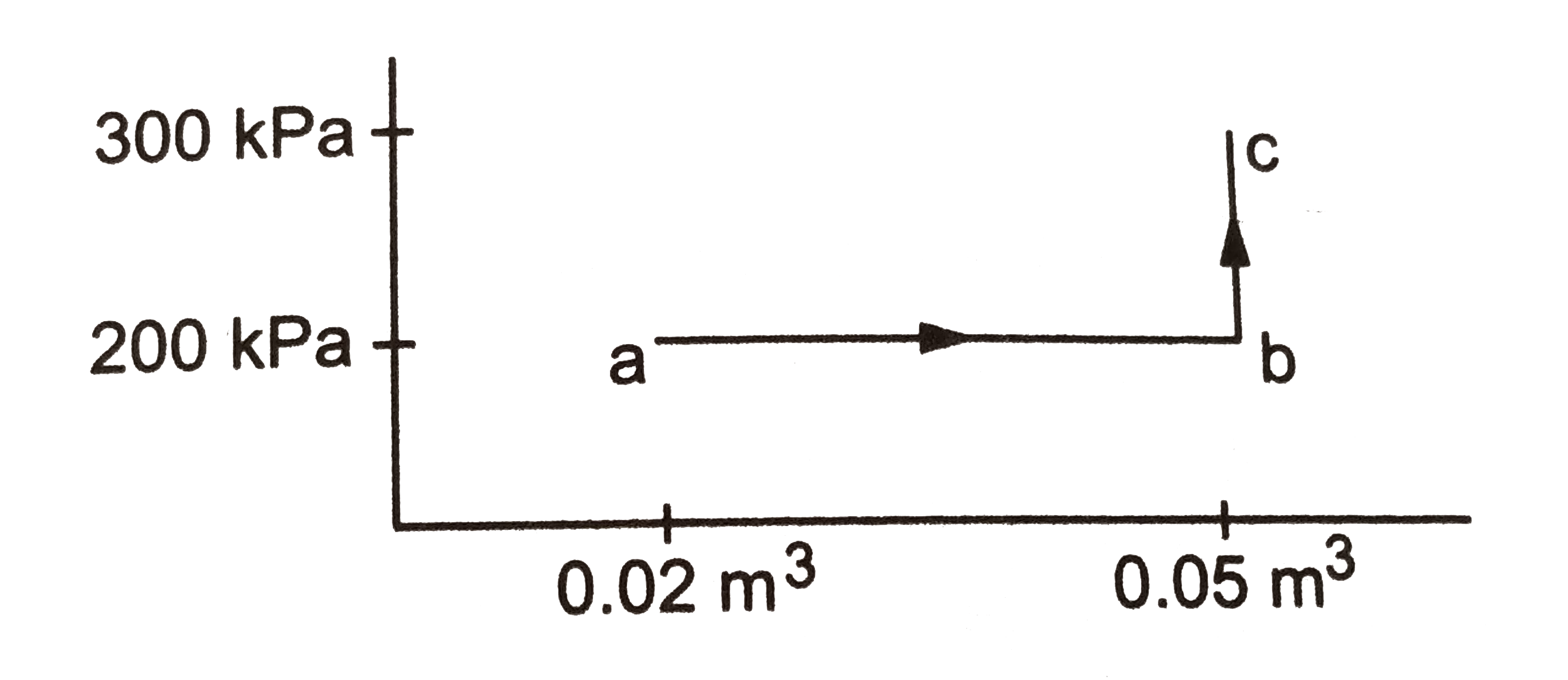
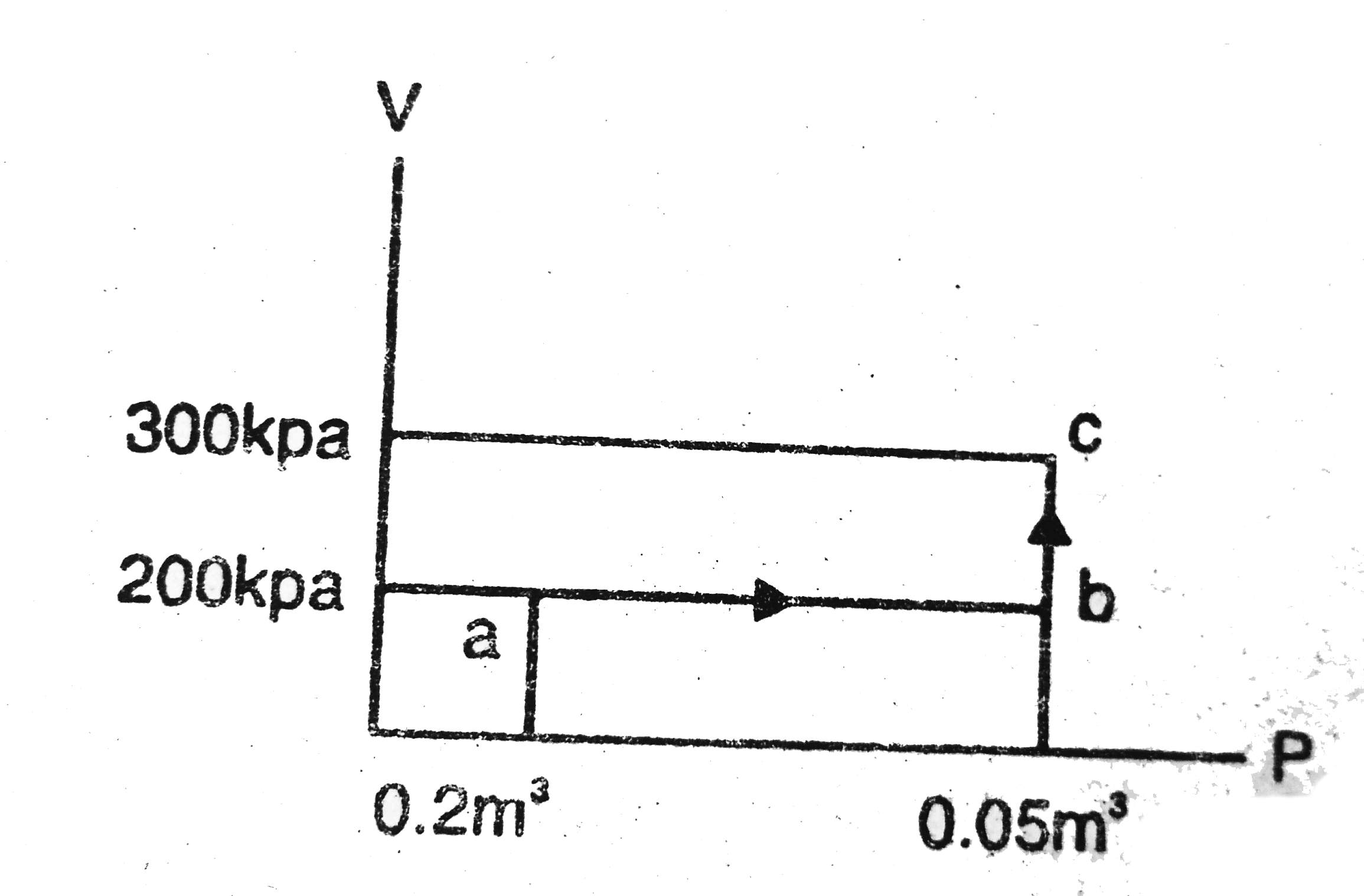
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HC VERMA ENGLISH-LAWS OF THERMODYNAMICS-All Questions
- calculate the heat absorbed by a system in going through the cyclic pr...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A substance is taken through the process abc as shown in fig, if the ...
Text Solution
|
- A gas is taken along the path AB as shown in fig, if 70 cal of heat is...
Text Solution
|
- The internal energy of a gas is given by U=1.5pV. It expands from 100...
Text Solution
|
- A gas is enclosed in a cylindrical vessel fitted with a frictionless p...
Text Solution
|
- A gas is initially at a pressure of 100 kPa and its volume is 2.0 m^(3...
Text Solution
|
- Consider the cyclic process ABCA, shown in fig, performed on a sample ...
Text Solution
|
- Fig shows the variation in the internal energy U with the volume V of ...
Text Solution
|
- Find the change in the internal energy of 2 kg of water as it heated f...
Text Solution
|
- Calculate the increase in the internal energy of 10 g of water when it...
Text Solution
|
- Fig shows a cylindrical tube of volume V with adiabatic walls containi...
Text Solution
|
- An adiabatic vessel of total volume V is divided into two equal parts ...
Text Solution
|
- Should the internal energy of a system necessarily increase if heat is...
Text Solution
|
- Should the internal energy of a system necessarily increase if its tem...
Text Solution
|
- A cylinder containing a gas is lifted from the first floor to the seco...
Text Solution
|
- A force F is applied on a block of mass M. The block is displaced thro...
Text Solution
|
- The outer surface of a cylinder containing a gas is rubbed vigorously ...
Text Solution
|
- When we rub our hands they become warm. Have we supplied heat to the h...
Text Solution
|
- A closed bottle contains some liquid. The bottle is shaken vigorously ...
Text Solution
|