Text Solution
Verified by Experts
HC VERMA ENGLISH-SPECIFIC HEAT CAPACITIES OF GASES-All Questions
- Two ideal gases have same value of (Cp / Cv = gamma). What will be the...
Text Solution
|
- A mixture contains 1 mole of helium (cp = 2.5 R, Cv 1.5 R. ) and 1mol...
Text Solution
|
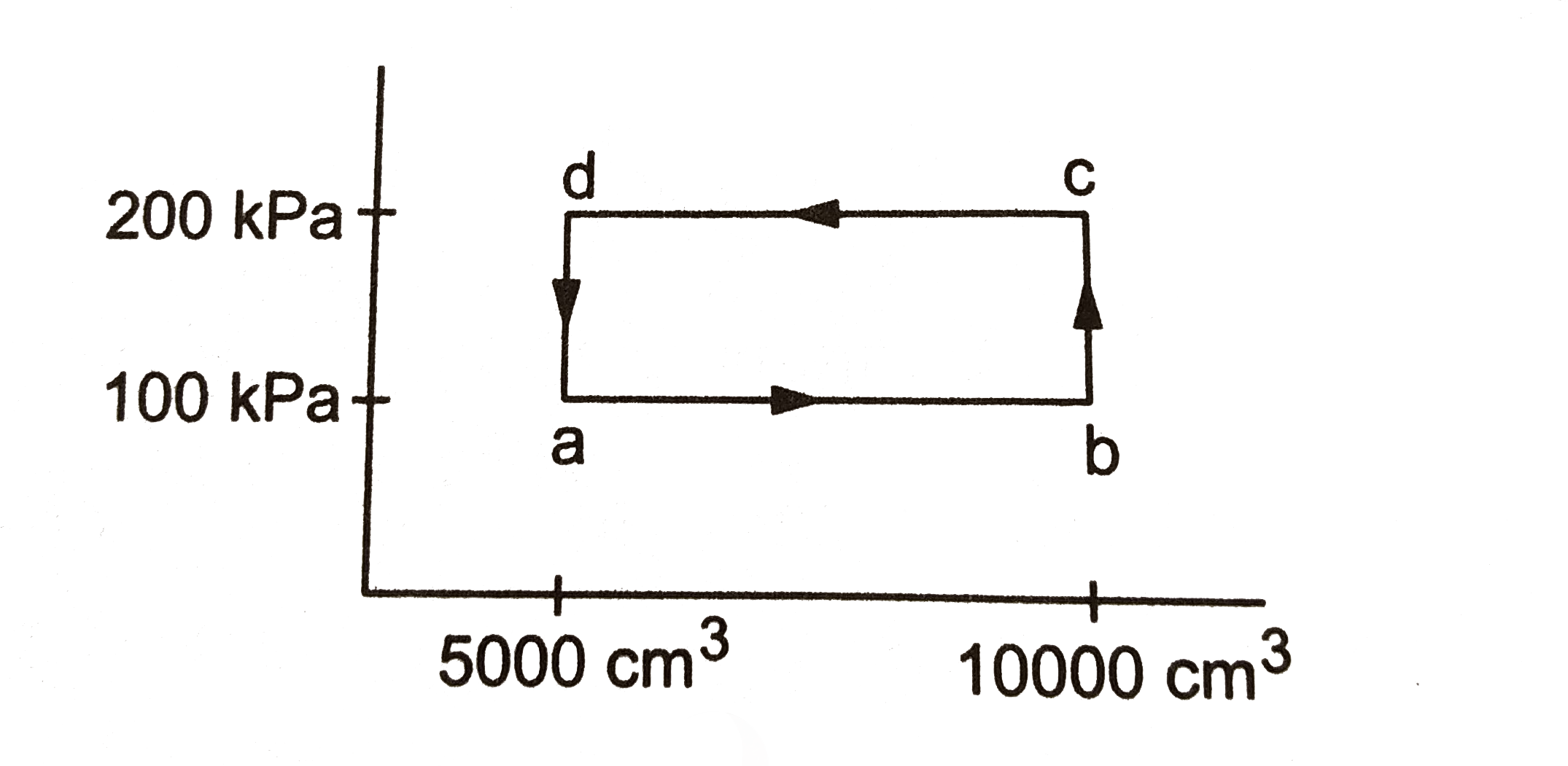
- Half mole of an ideal gas (gamma = 5/3) is taken through the cycle abc...
Text Solution
|
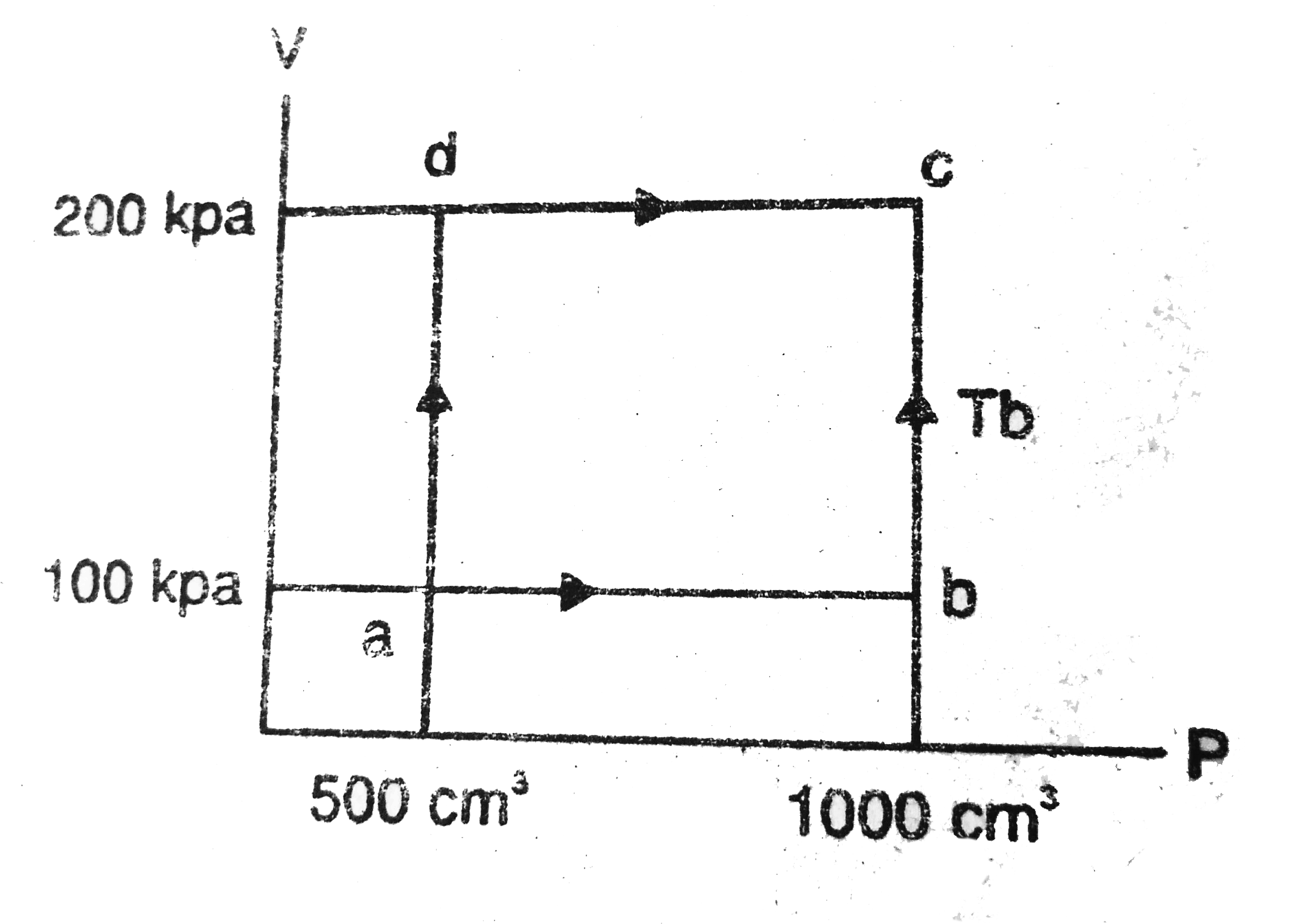
- An ideal gas (gamma = 1.67 ) is taken through the process abc shown in...
Text Solution
|
- In Joly's differential steam calorimeter, 3g of an ideal gas is cconta...
Text Solution
|
- The volume of an ideal gas (gamma = 1.5 ) is changed adiabatically fro...
Text Solution
|
- An ideal gas at pressure 2.5 xx 10^(5) pa and temperature 300k occupie...
Text Solution
|
- Air (gamma = 1.4 ) is pumped at 2atm pressure in a motor tyre at 20^@C...
Text Solution
|
- A gas is enclosed in a cylindrical can fitted with a piston. The walls...
Text Solution
|
- The initial pressure and volume of a given mass of a gas (Cp / Cv = ga...
Text Solution
|
- Conider a given sample of an ideal gas (Cp / Cv = gamma ) having initi...
Text Solution
|
- A given sample of an ideal gas (gamma = 1.5 ) is compressed adiabatica...
Text Solution
|
- Three samples A, B and C of the same gas (gamma = 1.5) have equal volu...
Text Solution
|
- Three samples A, B and C of the same gas (gamma = 1.5) have equal volu...
Text Solution
|
- Figure shows a cylindridcal tube with a adibatic walls and fitted with...
Text Solution
|
- In given figure, an adiabatic cylindrical tube of volume 2V(0) is divi...
Text Solution
|
- An adiabatic cylindrical tube of cross-sectional area 1 cm^(2) is clos...
Text Solution
|
- The speed of sound in hydrogen at 0^@C is 1280 m s^(-1). The density o...
Text Solution
|
- 4.0 g of helium occupies 22400 cm^(3) at STP. The specific heat capaci...
Text Solution
|
- An ideal gas having density 1.7 xx 10^(-3) g cm ^(-3) at a pressure 1....
Text Solution
|