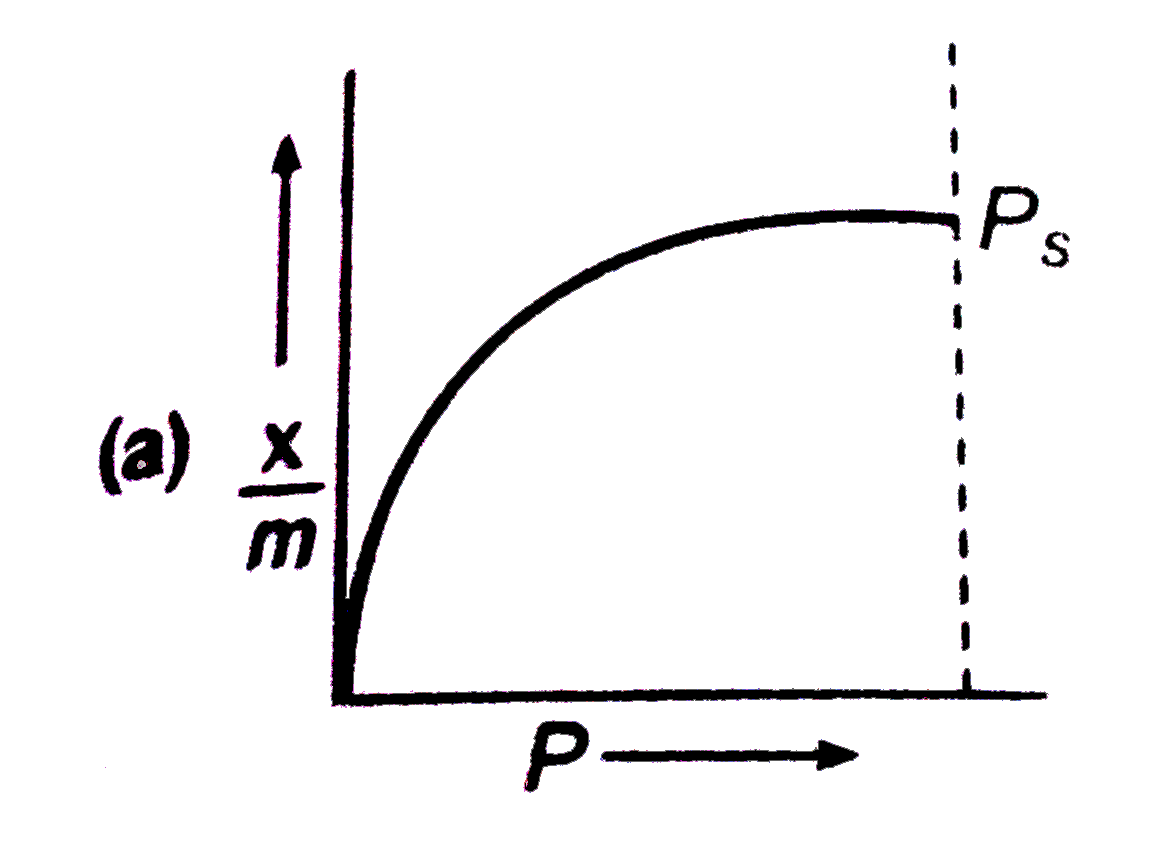
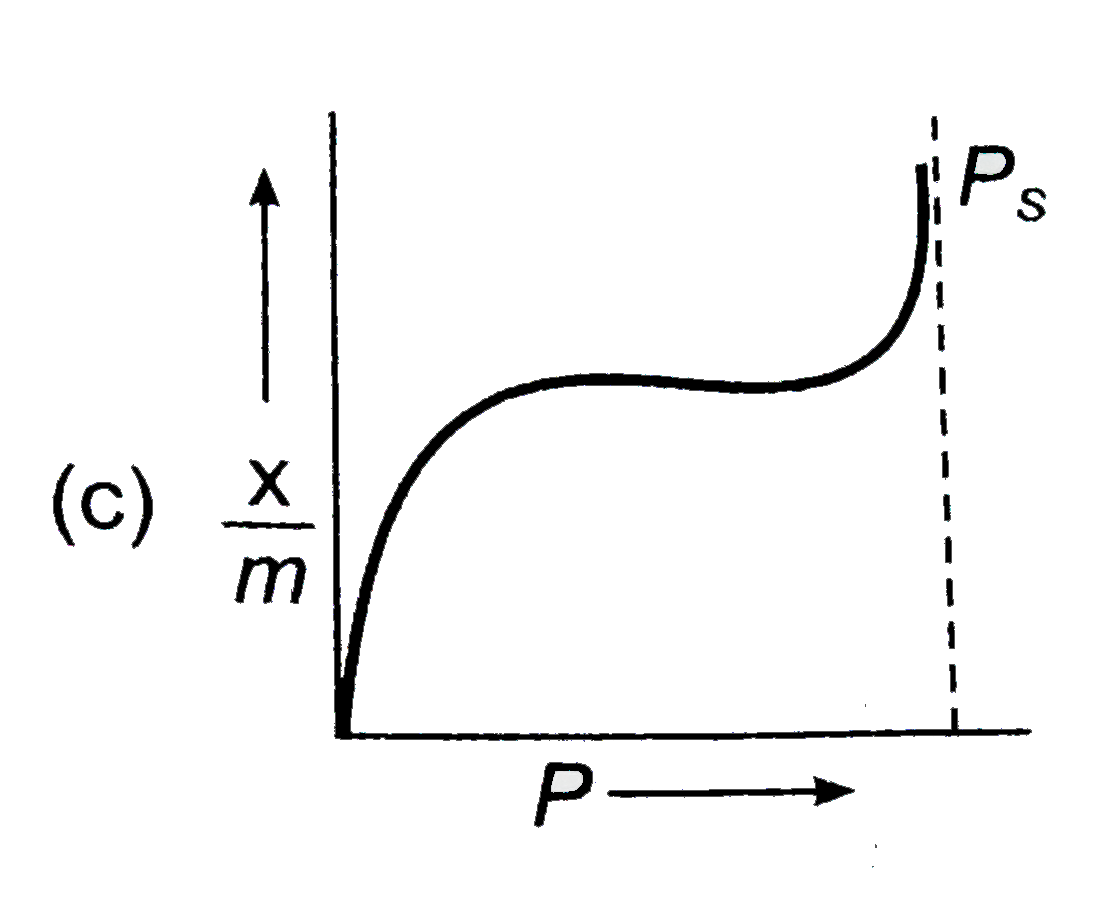
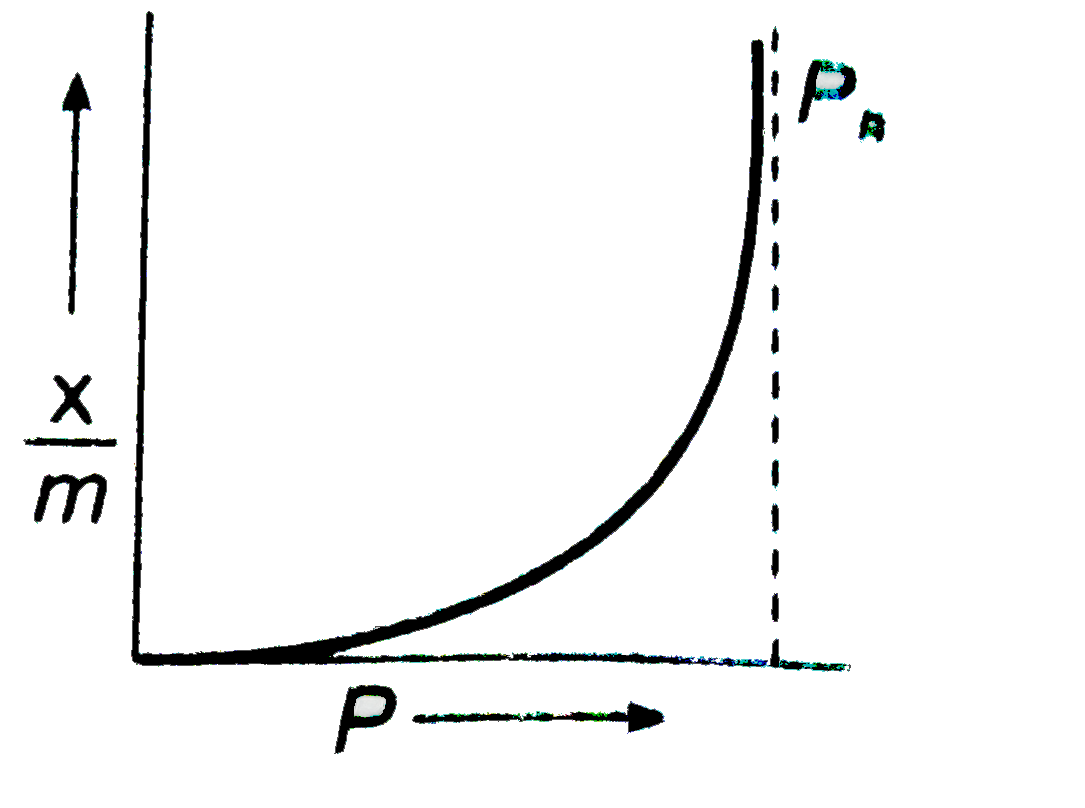
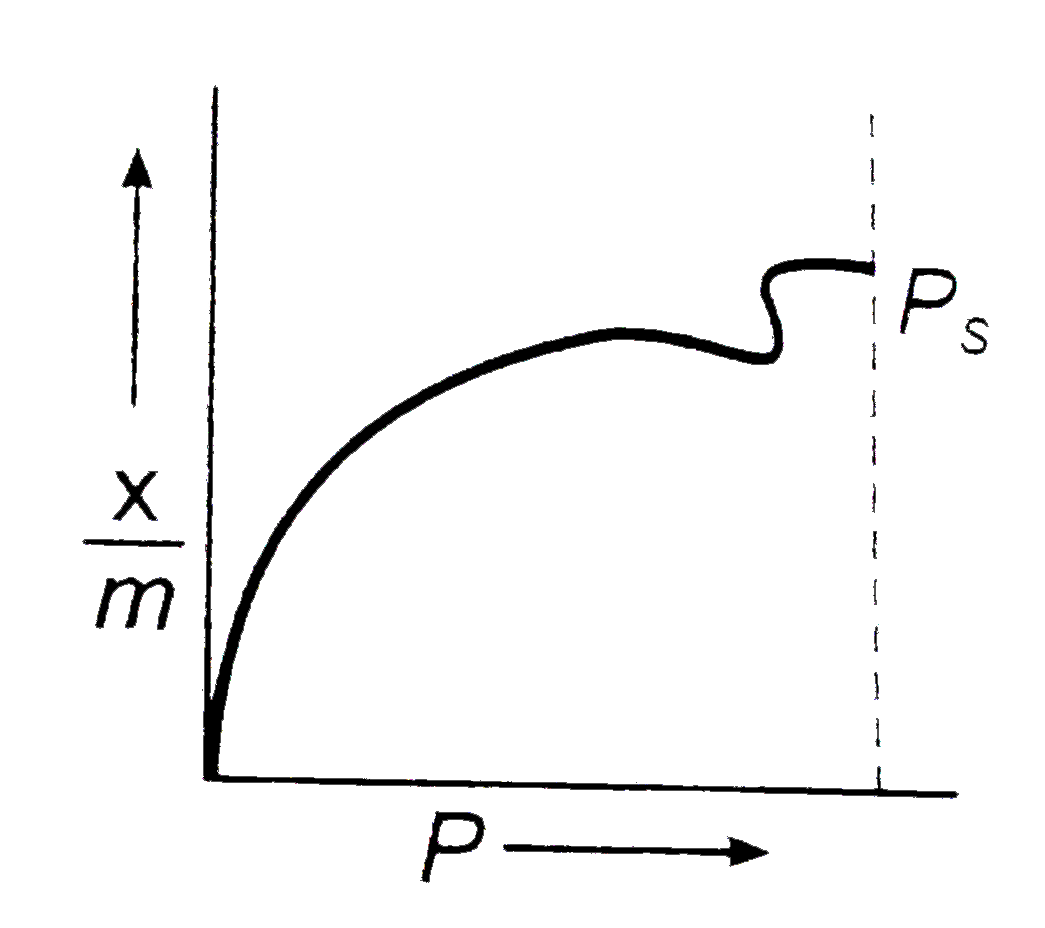A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- which of the following adsorption isotherms represents the adsorption...
Text Solution
|
- which of the following adsorption isotherms represents the adsorption...
Text Solution
|
- Which of the following graphs represents freundlich adsorption isother...
Text Solution
|
- Which of the following does not represent freundlich adsorption isot...
Text Solution
|
- In the adsorption of a gas on soild, Freundlich isotherm is obeyed . T...
Text Solution
|
- Adsorption isotherms are properly represented as in
Text Solution
|
- In Freundlich adsorption isotherm, adsorption is proportional to press...
Text Solution
|
- Which of the following graph represents Freundlich adsorption isotherm
Text Solution
|
- which of the following adsorption isotherms represents the adsorption...
Text Solution
|