select correct absorption isobars for chemisoption and physisoption respectivly ,
(where `x/m ` = extent of adsorption , T = temperature )
select correct absorption isobars for chemisoption and physisoption respectivly ,
(where `x/m ` = extent of adsorption , T = temperature )
(where `x/m ` = extent of adsorption , T = temperature )
A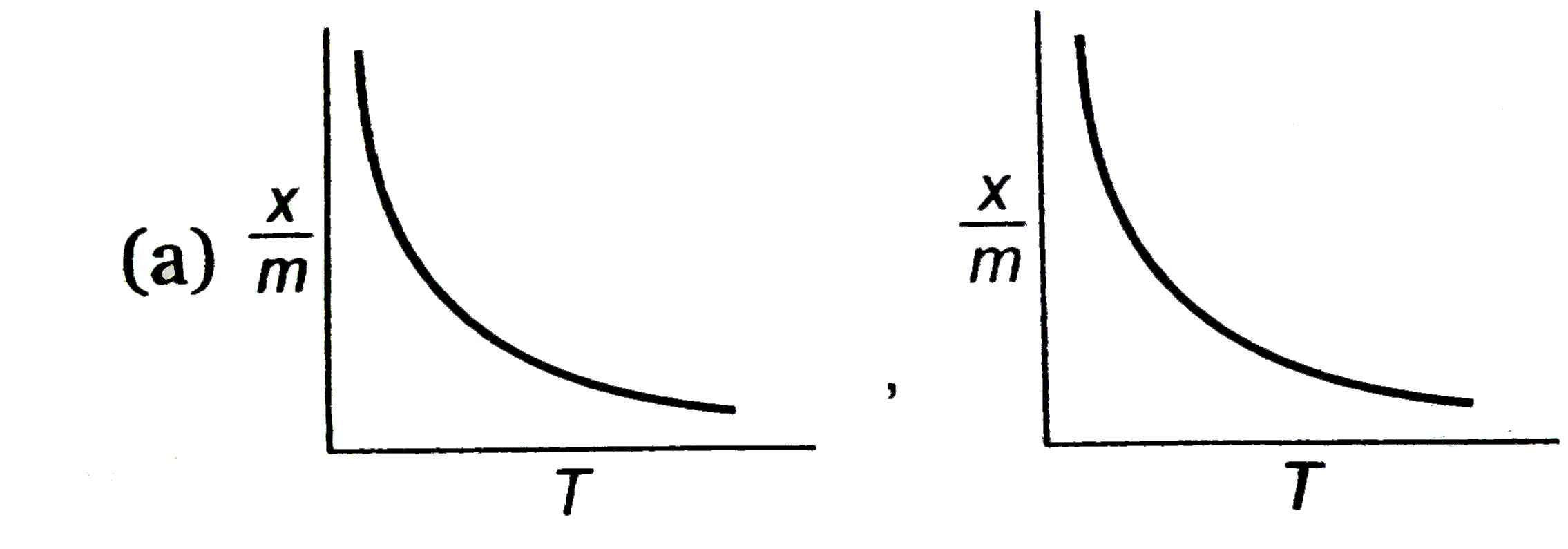
B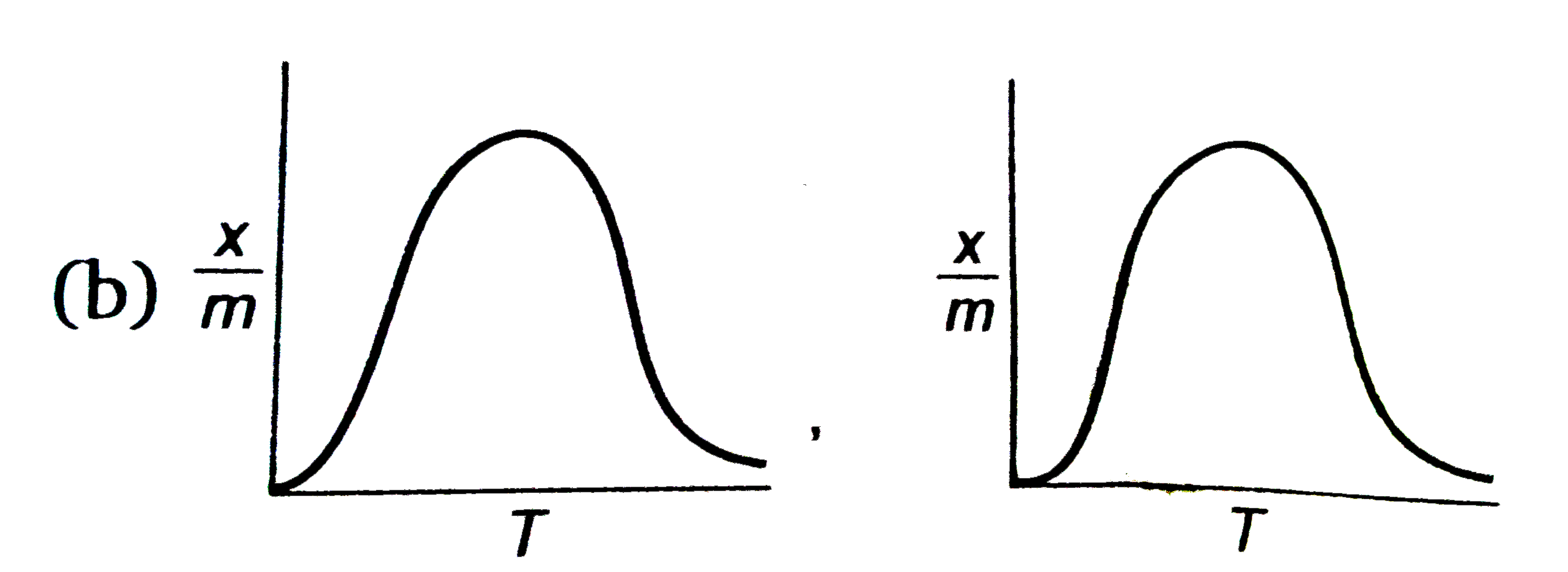

C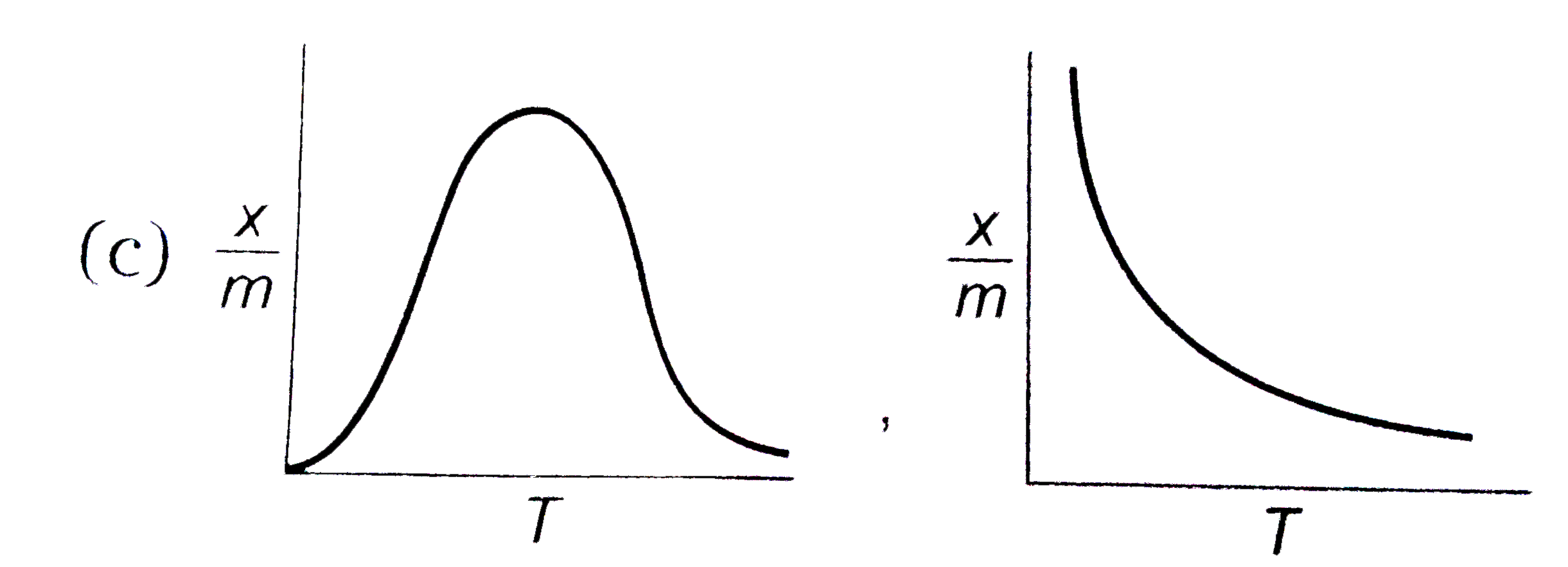
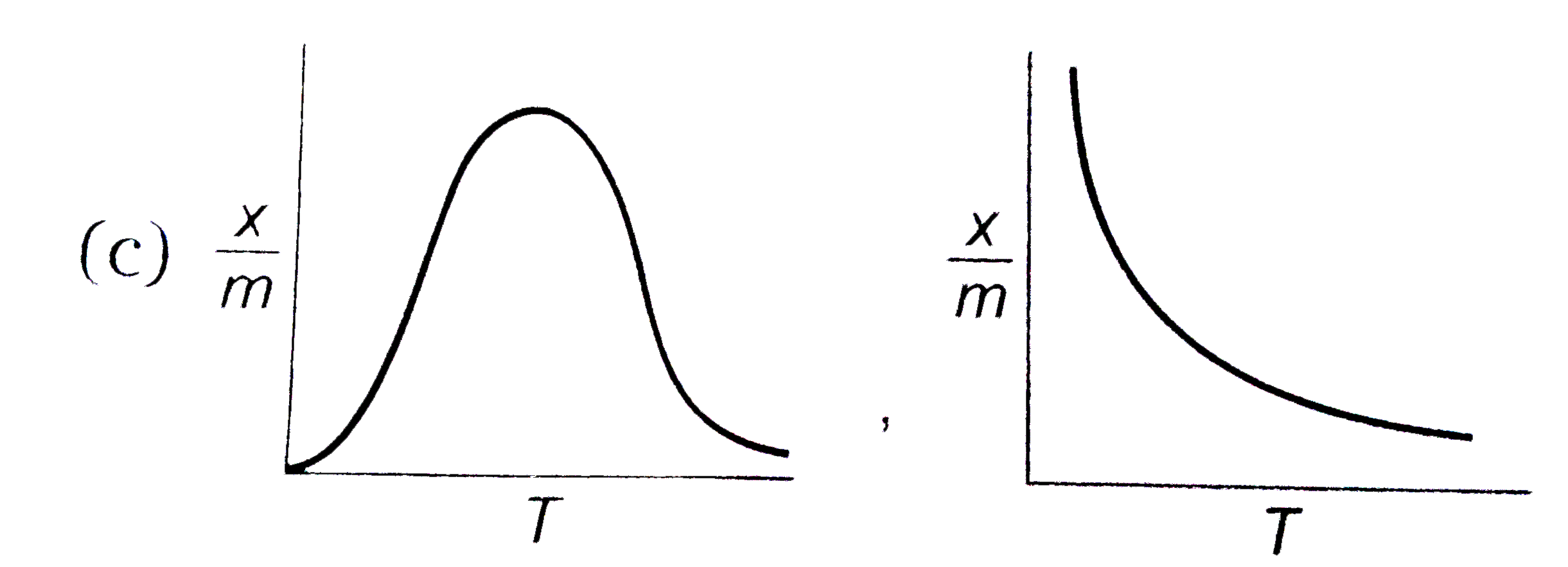
D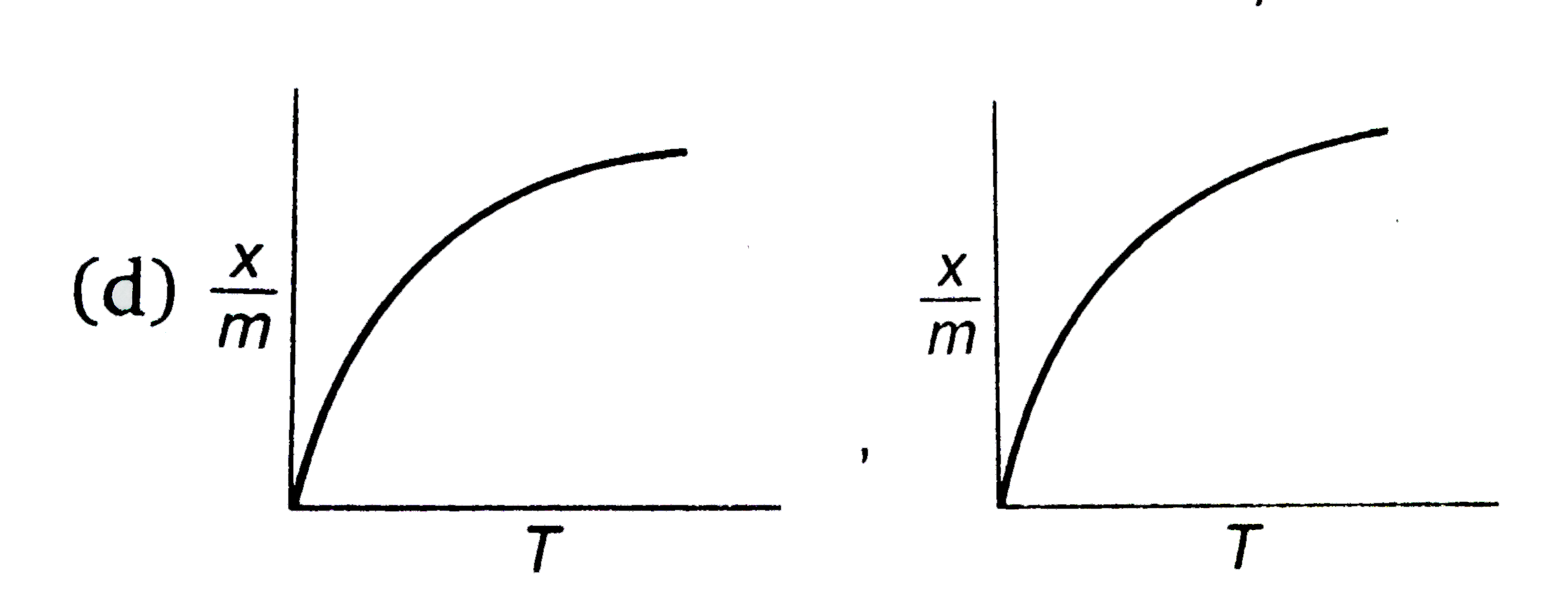
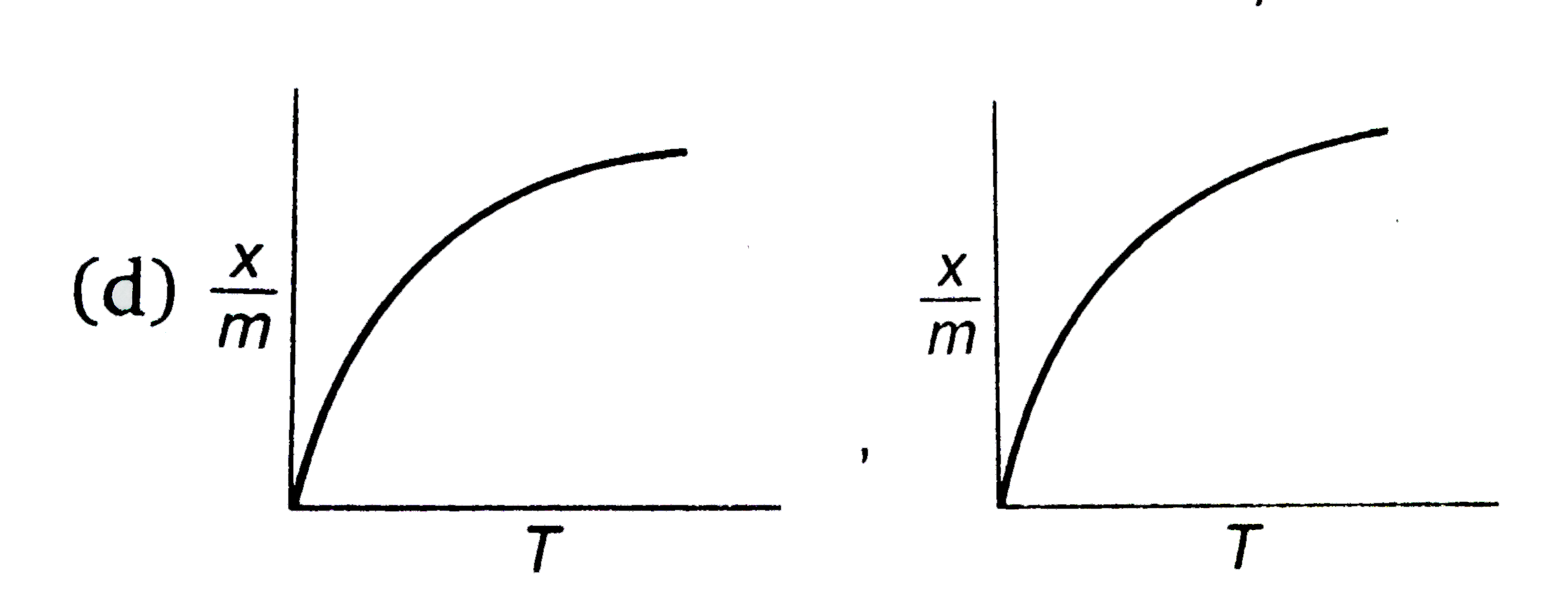
Text Solution
AI Generated Solution
The correct Answer is:
To determine the correct absorption isobars for chemisorption and physisorption, we need to analyze how the extent of adsorption (x/m) varies with temperature (T) under constant pressure conditions.
### Step-by-Step Solution:
1. **Understanding Chemisorption**:
- Chemisorption involves the formation of strong chemical bonds between the adsorbate (the substance being adsorbed) and the adsorbent (the surface onto which it is adsorbed).
- Initially, as the temperature increases, the kinetic energy of the molecules increases, which can enhance the extent of adsorption (x/m) because more molecules can overcome the energy barrier to form bonds.
2. **Effect of Temperature on Chemisorption**:
- However, at higher temperatures, the increased molecular motion can lead to bond breaking, which decreases the extent of adsorption.
- Therefore, the graph for chemisorption will initially rise with temperature and then fall after reaching a peak. This indicates that there is an optimal temperature range for chemisorption.
3. **Understanding Physisorption**:
- Physisorption involves weak van der Waals forces between the adsorbate and adsorbent. This process is generally exothermic, meaning that it releases energy when adsorption occurs.
- As the temperature increases, the thermal energy can disrupt these weak interactions, leading to a decrease in the extent of adsorption.
4. **Effect of Temperature on Physisorption**:
- For physisorption, as the temperature increases, the extent of adsorption (x/m) decreases continuously because the thermal energy overcomes the weak forces holding the adsorbate to the adsorbent.
- Thus, the graph for physisorption will show a continuous decline in the extent of adsorption with increasing temperature.
5. **Selecting the Correct Isobars**:
- Based on the above analysis, we can conclude:
- For chemisorption, the graph will initially increase and then decrease (indicating a peak).
- For physisorption, the graph will continuously decrease with increasing temperature.
- Therefore, the correct absorption isobars for chemisorption and physisorption respectively would be the one that shows these characteristics.
### Conclusion:
The correct absorption isobars for chemisorption and physisorption are:
- Chemisorption: Initially increases and then decreases with temperature.
- Physisorption: Continuously decreases with increasing temperature.
Similar Questions
Explore conceptually related problems
Consider the adsorption isotherm given below and interpret the variation in the extent of adsorption (x/m) when a. Temperature increased at constant pressure. b. Pressure increases at constant temperature.
Select the correct statements . (i) Physical adsorption is weak, multilayer, non-directional and non-specific. (ii) Chemical adsorption is strong, unilayer, directional and strong (iii) Chemical adsorption decreases with temperature (iv) Chemical adsorption is more stronger than physical adsorption
A rod of length l with thermally insulated lateral surface consists of material whose heat conductivity coefficient varies with temperature as k= a//T , where a is a constant. The ends of the rod are kept at temperatures T_1 and T_2 . Find the function T(x), where x is the distance from the end whose temperature is T_1 .
Observe the given adsorption isotherm carefully and choose the correct option (i) These curves indicates that at a fixed temperature, there is a decrease in physical adsorption with increase in pressure. (ii) These curves always seem to approach saturation at high pressure. x/m=k*p^(1//n) (n gt 1) is generally represented by this isotherm. (iv) These curves indicate that at a fixed pressure, there is a decrease in physcial adsorption with increase in temperature .
A chemist studied the phenomenon of adsorption by putting blood charcoal in KCL solution. He observed difference in the behaviour with dilute KCL solution and with concentrated KCL solution. He also studied the adsorption of different gases on solid adsorbent and the effect of temperature on adsorption. He put forward a mathematical relationship relating x//m with equilibrium pressure. The correct order of the adsorption of gases will be
A chemist studied the phenomenon of adsorption by putting blood charcoal in KCL solution. He observed difference in the behaviour with dilute KCL solution and with concentrated KCL solution. He also studied the adsorption of different gases on solid adsorbent and the effect of temperature on adsorption. He put forward a mathematical relationship relating x//m with equilibrium pressure. Which of the following is correct ?
A chemist studied the phenomenon of adsorption by putting blood charcoal in KCL solution. He observed difference in the behaviour with dilute KCL solution and with concentrated KCL solution. He also studied the adsorption of different gases on solid adsorbent and the effect of temperature on adsorption. He put forward a mathematical relationship relating x//m with equilibrium pressure. Which of the following plot will be liner? (More than one correct)
A particle is in motion on the x-axis. The variation ofits velocity with position is as shown. The graph is circle and its equation is x^(2)+v^(2)=1 , where x is in m and v in m/s. The correct statement(s) is/are:-
In Freundlich adsorption isotherm, at moderate pressure extent of adsorption (x/m) directly proportional to P^(x) . The value of x is
The equation of state for a gas is given by PV = eta RT + alpha V , where eta is the number of moles and alpha a positive constant. The intinal pressure and temperature of 1 mol of the gas contained in a cylinder is P_(0) and T_(0) , respectively. The work done by the gas when its temperature doubles isobarically will be
Recommended Questions
- select correct absorption isobars for chemisoption and physisoption re...
Text Solution
|
- select correct absorption isobars for chemisorption and physisorption ...
Text Solution
|
- Select correct adsorption isobars for chemisorption and physisorption ...
Text Solution
|
- Which plot is the adsorption isobar for chemisorption where x is...
Text Solution
|
- Which plot is the adsorption isobar for chemisorption where x is the a...
Text Solution
|
- Select correct adsorption isobars for chemisorption and physisrption r...
Text Solution
|
- विलयनों में, अधिशोषण की सीमा, ताप बढ़ाने पर
Text Solution
|
- select correct absorption isobars for chemisoption and physisoption re...
Text Solution
|
- The following are some statements about adsorption of solutes from the...
Text Solution
|