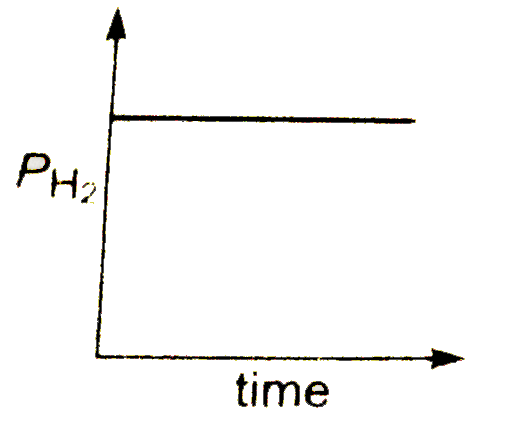
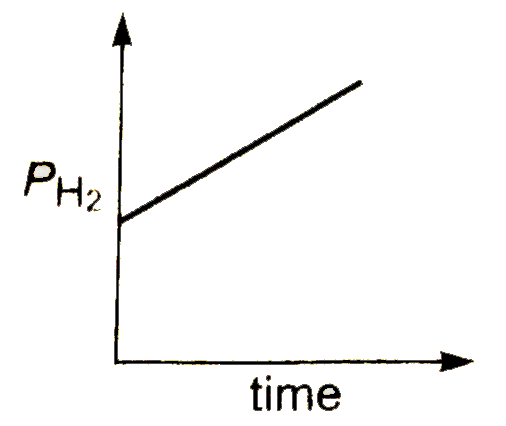
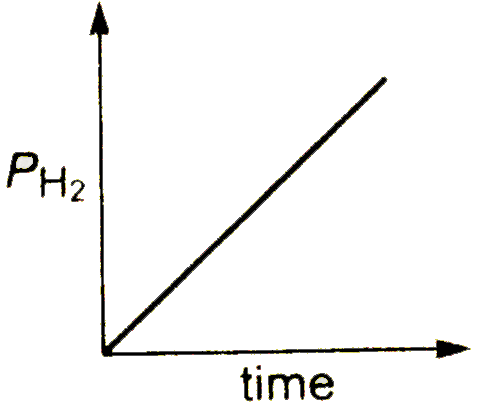
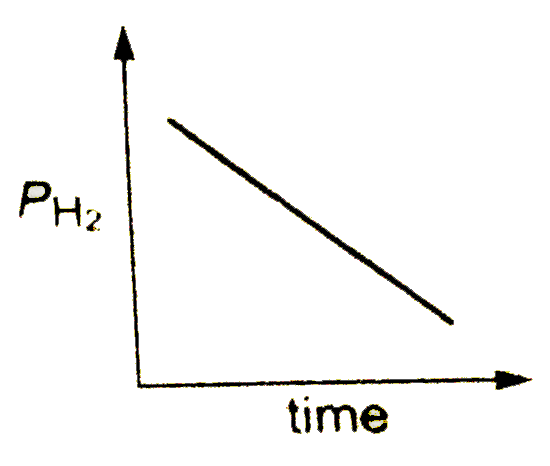
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Decomposition of Hl (g) on Gold surface is zero order reaction. Initia...
Text Solution
|
- Which of the following graphs are correct for the given reaction? H(...
Text Solution
|
- Decomposition of Hl (g) on Gold surface is zero order reaction. Initia...
Text Solution
|
- For the decomposition of Hl on the surface of gold the only correct co...
Text Solution
|
- Which of the following graphs is correct for a zero order of reaction?
Text Solution
|
- Assertion : The decomposition of gaseous ammonia on a hot platinum sur...
Text Solution
|
- Which of the following graphs is correct for a zero order reaction ?
Text Solution
|
- Which of the following graphs is correct for a zero order reaction -
Text Solution
|
- Which of the following graph is correct for zero order reaction?
Text Solution
|