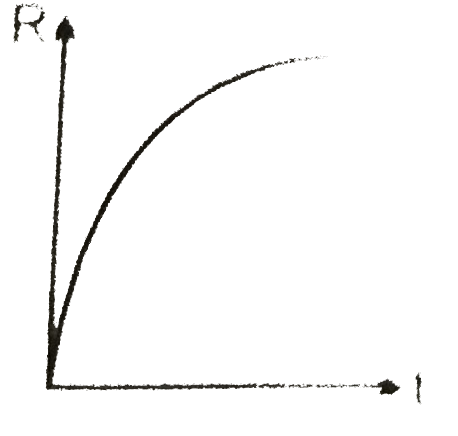
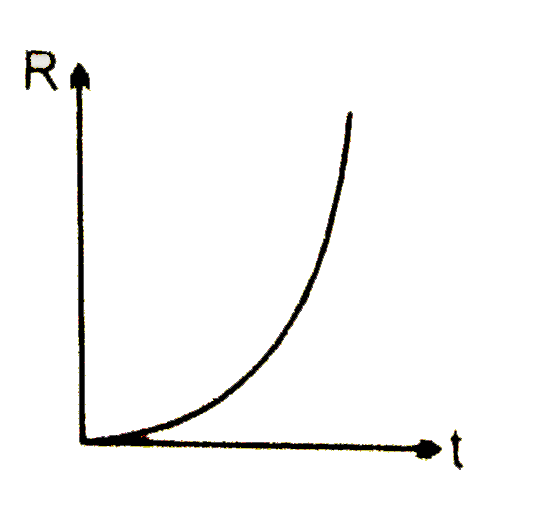
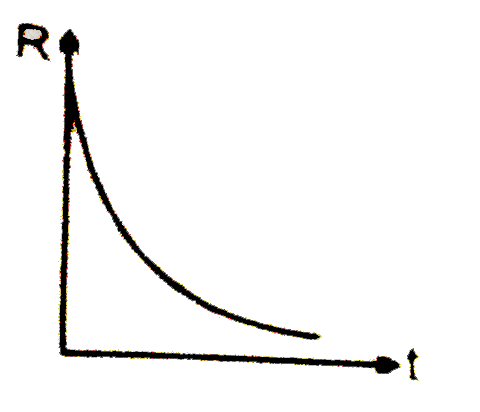
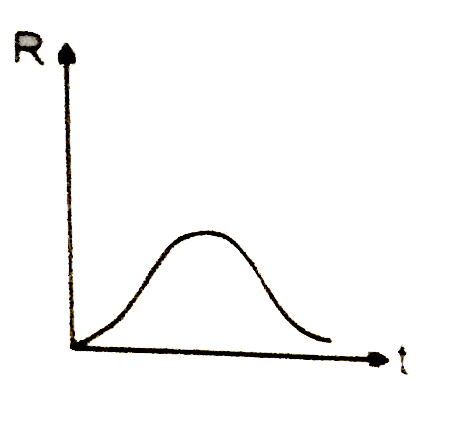
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If decompositon reaction A(g)toB(g) follows first order linetics then ...
Text Solution
|
- A radioactiev nuleus X deays to a stable nuleus Y . Then, time graph o...
Text Solution
|
- Which graph represents zero-order reaction [A(g) rarr B (g)] ?
Text Solution
|
- If decompositon reaction A(g)toB(g) follows first order linetics then ...
Text Solution
|
- In the reaction PCl(5)(g)hArrPCi(3)(g)+Ci(2)(g) a graph in plotted...
Text Solution
|
- For a first order gas phase reaction : A((g)) to 2B((g)) +C((g)) ...
Text Solution
|
- Give two examples for each of zero order and first order reactions. Wr...
Text Solution
|
- For the first order gas phase decomposition reaction, A(g) to B(g) +...
Text Solution
|
- Consider a first order gas phase decomposition reaction given below : ...
Text Solution
|