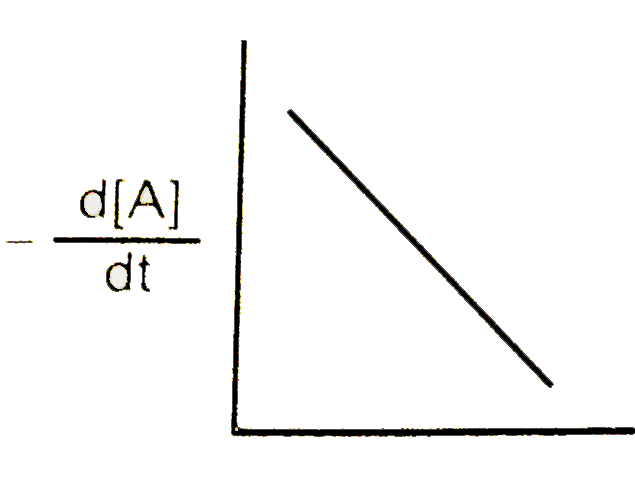
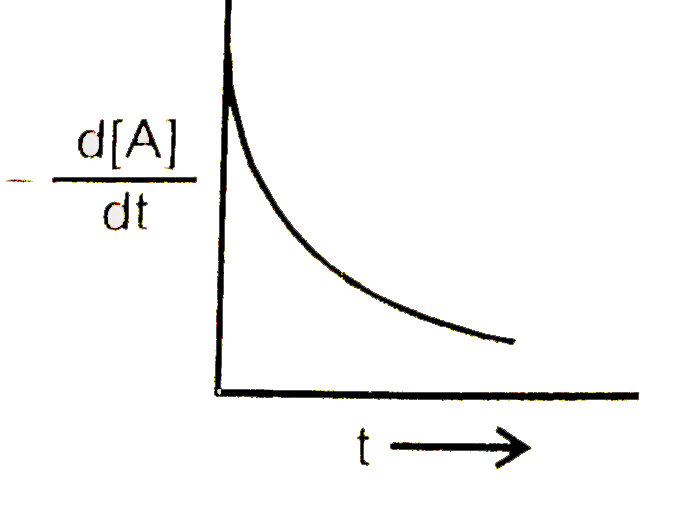
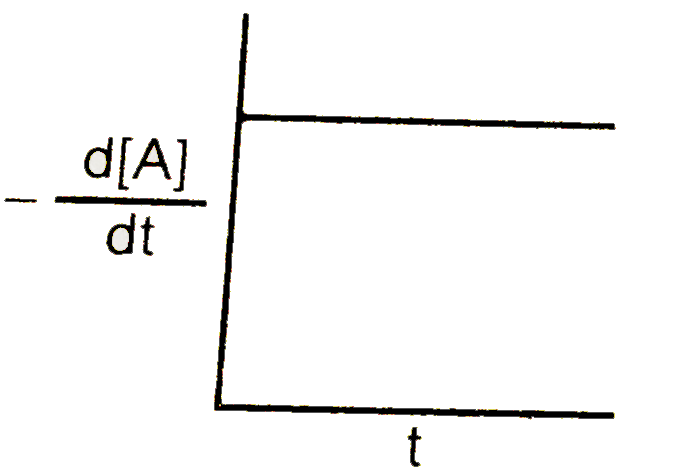
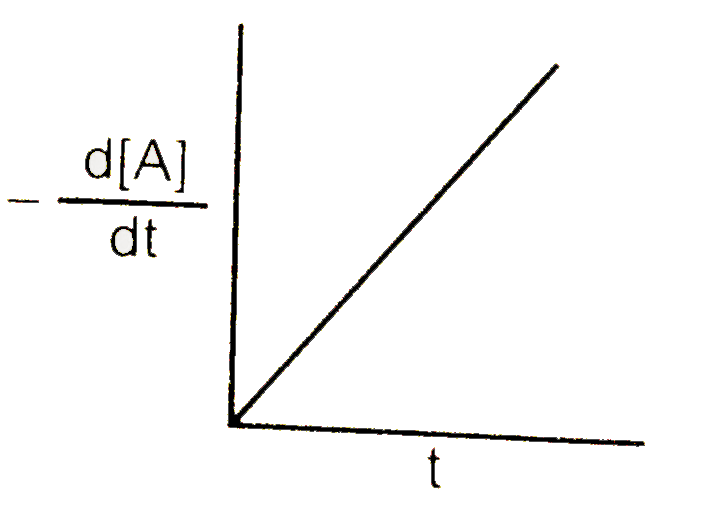
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reactionAtoB, for which graph between half life (t(1//2)) and ...
Text Solution
|
- The graph between concentration (X) of the Product and time of the rea...
Text Solution
|
- Following is the graph between log T(50) and log a ( a = initial conce...
Text Solution
|
- Following is the graph between log t(1//2) and log a (a initial concen...
Text Solution
|
- For which order reaction a straight line is obtained along with x -axi...
Text Solution
|
- A nucleus A (parent) decays into B (Daughter) with half life T(1) and ...
Text Solution
|
- For the reaction AtoB , for which graph between half life (t(1//2)) an...
Text Solution
|
- Graph between concentration of the product and time of the reaction A ...
Text Solution
|
- Following is the graph between log T(50) and log a (a = initial concen...
Text Solution
|