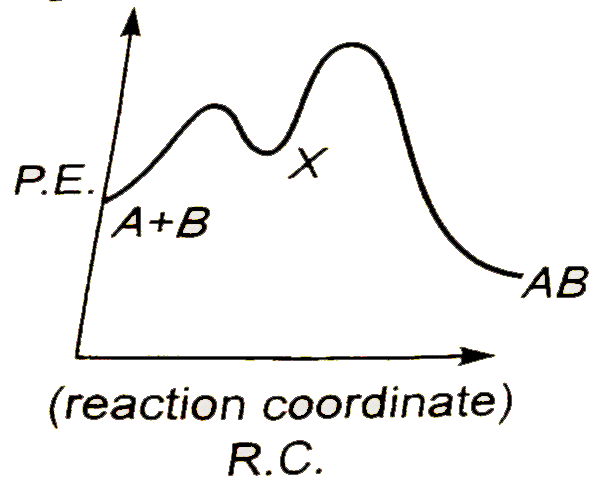
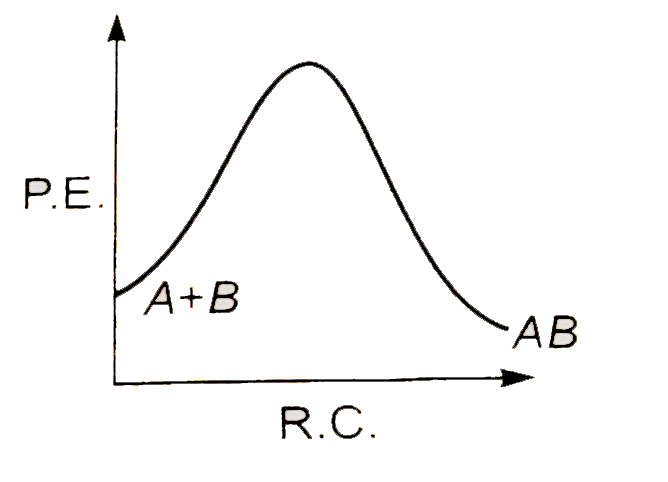
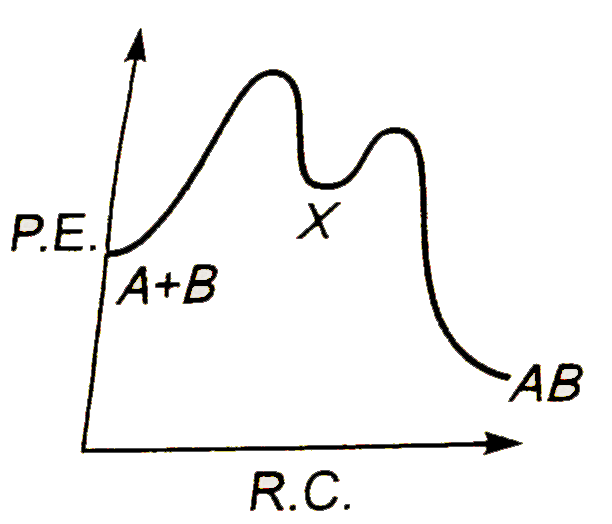
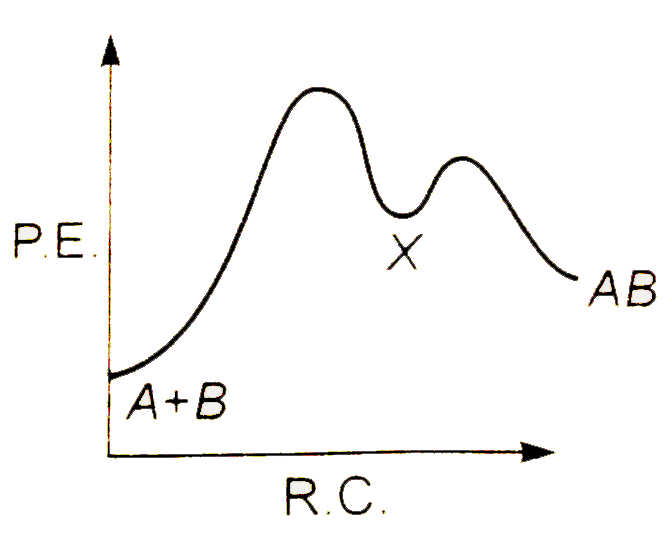
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|
- The following reaction is accompanied by liberation of heat and occurs...
Text Solution
|
- For an exothermic chemical process occuring in 2 steps as (i) A+B rarr...
Text Solution
|
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|
- A chemical process occurring in two steps , is plotted as
Text Solution
|
- A chemical reaction proceeds into the following steps Step I, 2A hAr...
Text Solution
|
- For an exothermic chemical process occurring in two steps as (i) A+B...
Text Solution
|
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|