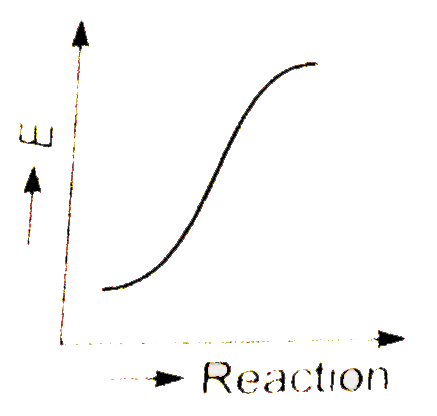
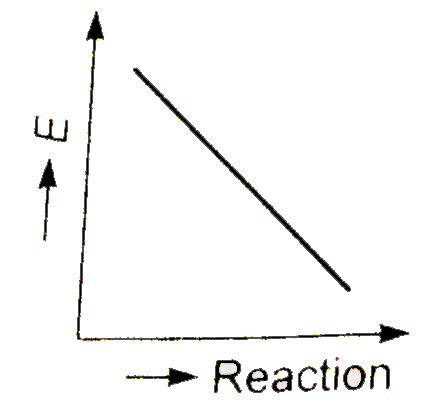
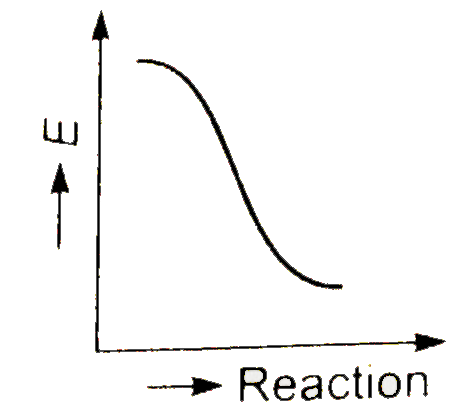
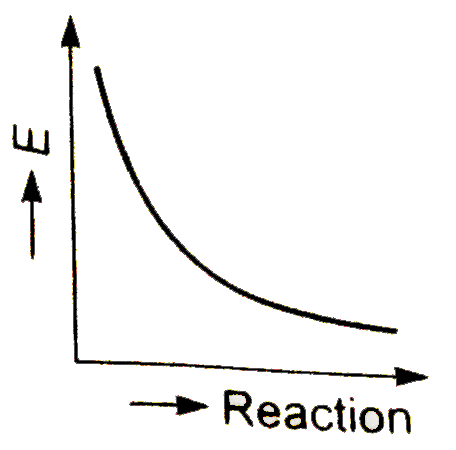
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which graph shows zero activation energy ?
Text Solution
|
- Can activation energy for reactions be zero?
Text Solution
|
- Which of the following reactions has Zero activation energy?
Text Solution
|
- Which graph shows zero activation energy ?
Text Solution
|
- Draw a graph of potential energy v/s reaction co-ordinate showing the ...
Text Solution
|
- ऊष्माक्षेपी अभिक्रिया के लिए सक्रियण ऊर्जा दर्शाने वाला ग्राफ दीजिये।
Text Solution
|
- अभिक्रिया (reaction) के लिए कौन-सा ग्राफ शून्य सक्रियण ऊर्जा दर्शाता ह...
Text Solution
|
- Derive an integrated rate equation for the rate constant of a zero ord...
Text Solution
|
- अभिक्रिया के लिए कौनसा ग्राफ शून्य सक्रियण ऊर्जा दर्शाता है
Text Solution
|