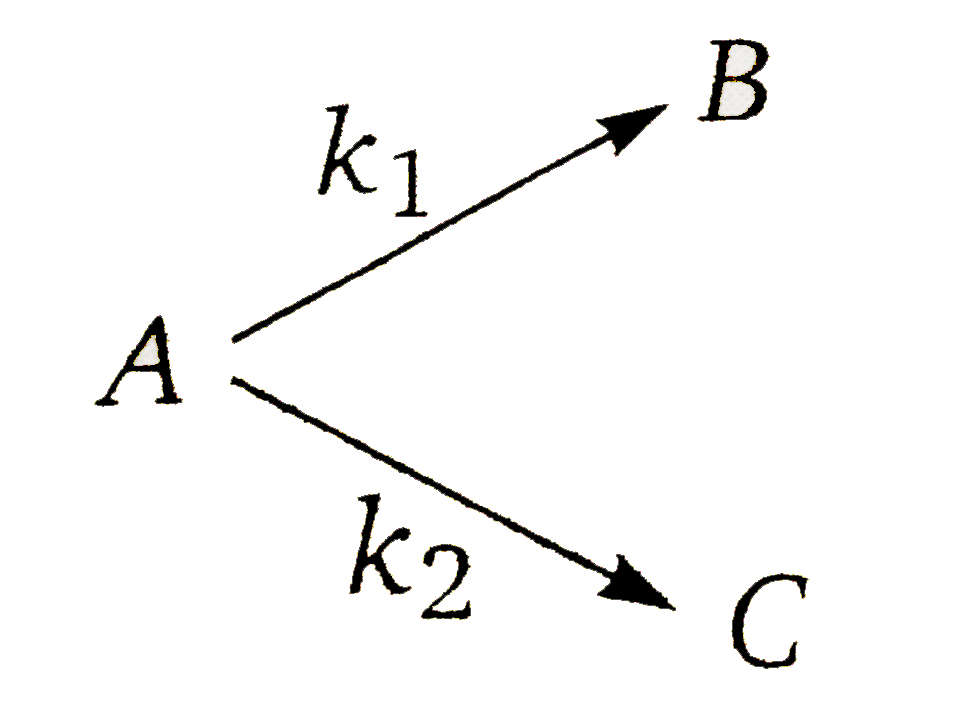
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the reaction. The rate constant for two parallel reacti...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- Consider the reaction. The rate constant for two parallel reacti...
Text Solution
|
- The substance undergoes first order decomposition.The decomposition fo...
Text Solution
|
- The rate constant for two parallel reactions were found to be 1.0xx10^...
Text Solution
|
- The unit of rate constant of a reaction is ("mol")^(-1//2). ("dm")^(3/...
Text Solution
|
- The rate constant for two parallel reactions were found reactions were...
Text Solution
|
- The rate constants for a reaction at 400 K and 500 K are 2 . 60 xx 10^...
Text Solution
|
- For a chemical raction AtoB the rate of the reaction is 2xx10^(-3) mol...
Text Solution
|