Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- , At time t=0, intial mole of A is 1. Overall half time of the react...
Text Solution
|
- For a gaseous reaction, following data is given: ArarrB, k(1)= 10^(1...
Text Solution
|
- A reaction takes place in theee steps: the rate constant are k(1), k(2...
Text Solution
|
- For the consecutive unimolecular-type first-order reaction A overset(k...
Text Solution
|
- Two consecutive irreversible fierst order reactions can be represented...
Text Solution
|
- Two consecutive irreversible fierst order reactions can be represented...
Text Solution
|
- , At time t=0, intial mole of A is 1. Overall half time of the react...
Text Solution
|
- A reaction takes place in three steps. The rate constant are k(1), k(2...
Text Solution
|
- Write the equilibrium expressions (K(C )) for the decomposition of 2 m...
Text Solution
|
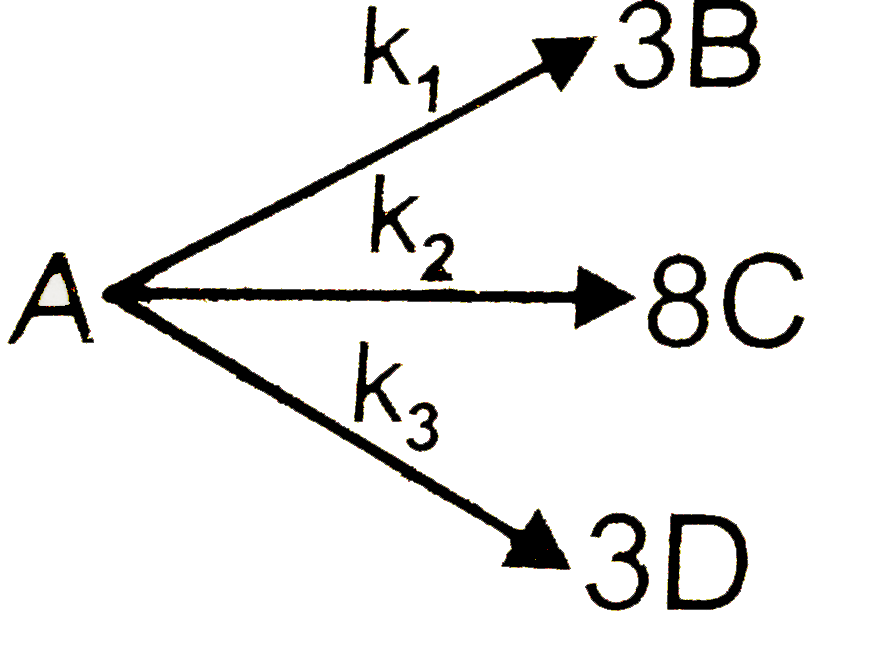 , At time t=0, intial mole of A is 1.
, At time t=0, intial mole of A is 1.