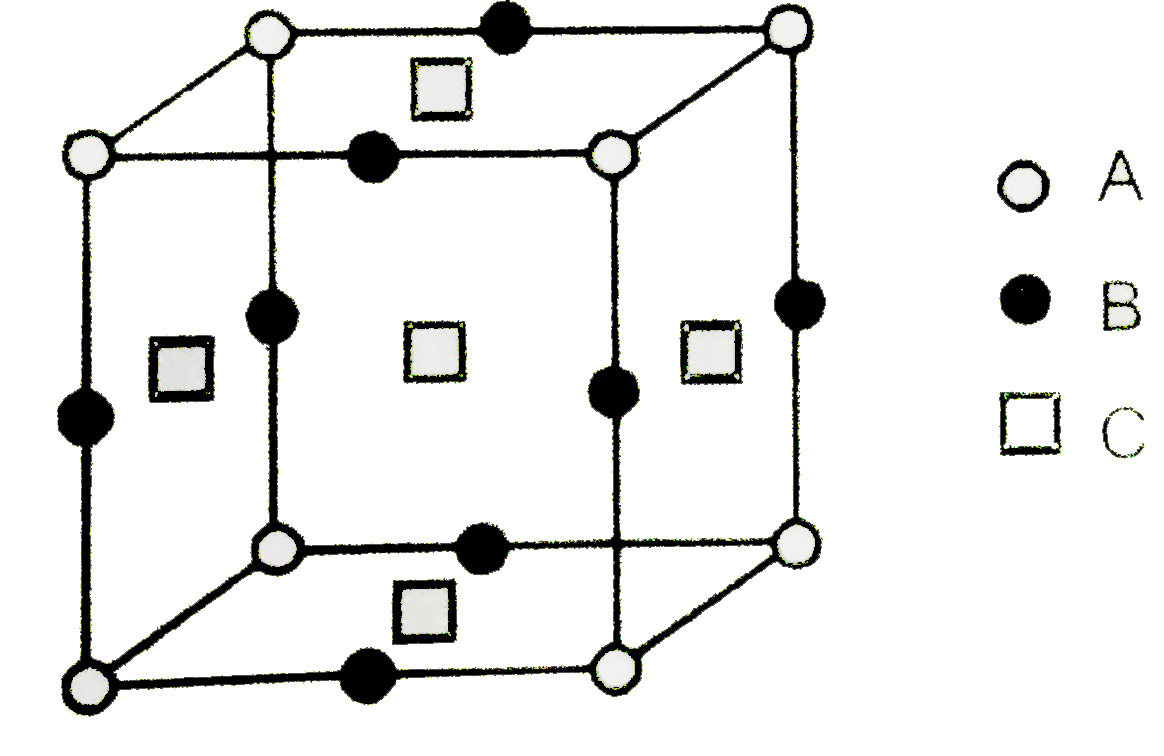
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
NARENDRA AWASTHI ENGLISH|Exercise Level 3 passage -1|1 VideosSOLID STATE
NARENDRA AWASTHI ENGLISH|Exercise Passge-2|4 VideosSOLID STATE
NARENDRA AWASTHI ENGLISH|Exercise Subjective Problems|13 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI ENGLISH|Exercise Match the Colum-II|6 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-SOLID STATE-Level-2
- First three nearest neighboure distance for primitive cubic lattice ...
Text Solution
|
- First three nearestneighbour distances for body centered cubic latti...
Text Solution
|
- Given : The unit cell structure o fcompound is show below . ...
Text Solution
|
- The density of apure substance 'A' whose atoms are in cubic close ...
Text Solution
|
- In a planar tetra - atomic molecule, XY(3),X is at the centroid of ...
Text Solution
|
- How many unit cells are present in 5.0 gm of crystal AB (formula m...
Text Solution
|
- The density of CaF(2) (flourtie structure ) is 3.18 g// cm^(3). The l...
Text Solution
|
- A crystal of lead (II) sulphide has NaCl strcuture . In this crys...
Text Solution
|
- CdO has NaCl like structure with density 8.27g//c c. If the ionic radi...
Text Solution
|
- KCl crystallizes int the same type of lattic as done NaCl . Given ...
Text Solution
|
- Ferrous oxide has a cubie structure and edge length of the uint ce...
Text Solution
|
- If an element (at. Mass =50) crystallise in fc lattie ,with a= 0.50...
Text Solution
|
- An element X (At. Wt. =24) forms FCC lattice. If the edge length of la...
Text Solution
|
- In fcc lattice ,A, B, C,D atoms are arranged at corner , face centre...
Text Solution
|
- The distance between an ocatahral and tetrahedral void in fcc lat...
Text Solution
|
- A(2)B molecules(" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- Graphite has h.c.p arrangements of carbon atoms and the parallel p...
Text Solution
|
- How many effiective Na^(+) and Cl^(-) ions are present respective...
Text Solution
|
- A crystal is made of particles X and Y.X form fcc packing and Y o...
Text Solution
|
- Select right expression for determinig packing fraction (P.F.) of Na...
Text Solution
|