A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- STATEMENT-1: The ground state electronic configuration of nitrogen is ...
Text Solution
|
- STATEMENT-1: The ground state electronic configuration of introgen is ...
Text Solution
|
- The above electronic configuration deviates from (I) Hund's rule (II) ...
Text Solution
|
- STATEMENT-1: The ground state electronic configuration of introgen is ...
Text Solution
|
- State Hund's rule and Aufbau principle.
Text Solution
|
- 'Moeller chart' deals with a) Pauli's principle b) Aufbau principle ...
Text Solution
|
- Electronic configuration of elements| Aufbau principle| Pauli exclusio...
Text Solution
|
- Questions based on Quantum numbers and Difference between orbit and or...
Text Solution
|
- Q.22 (b) :-The principle state that the value of four quantum numbers ...
Text Solution
|
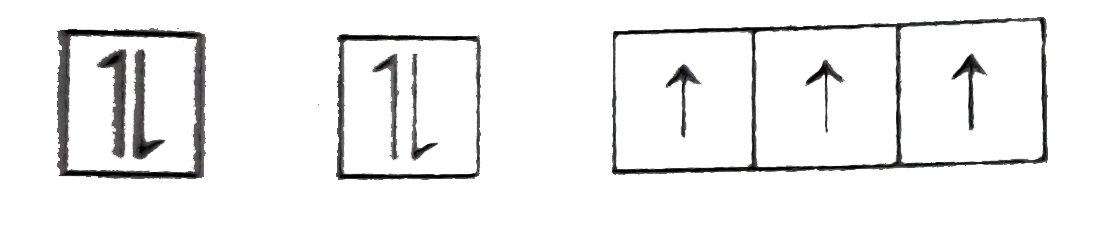 STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and pauli's principle.
STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and pauli's principle.