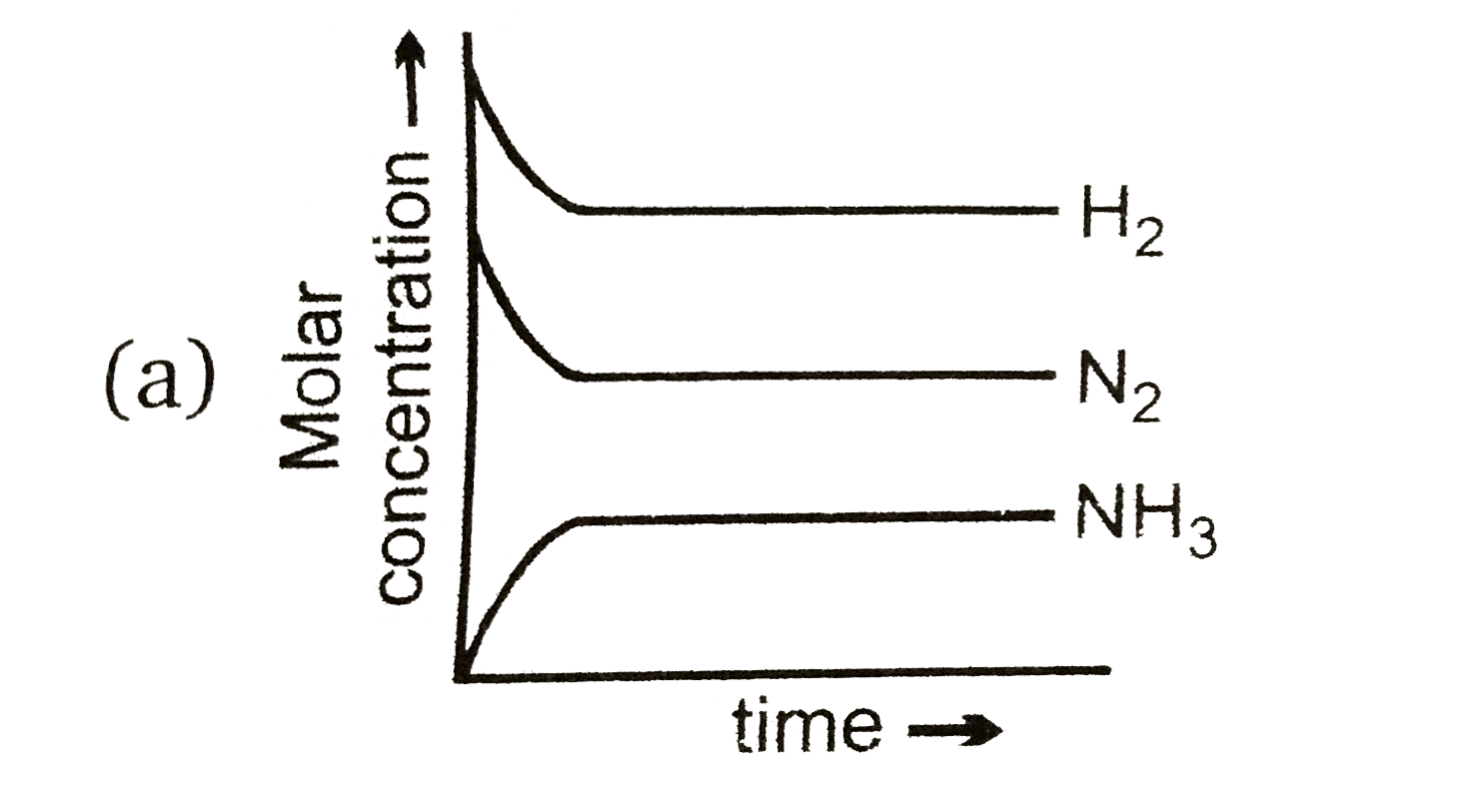
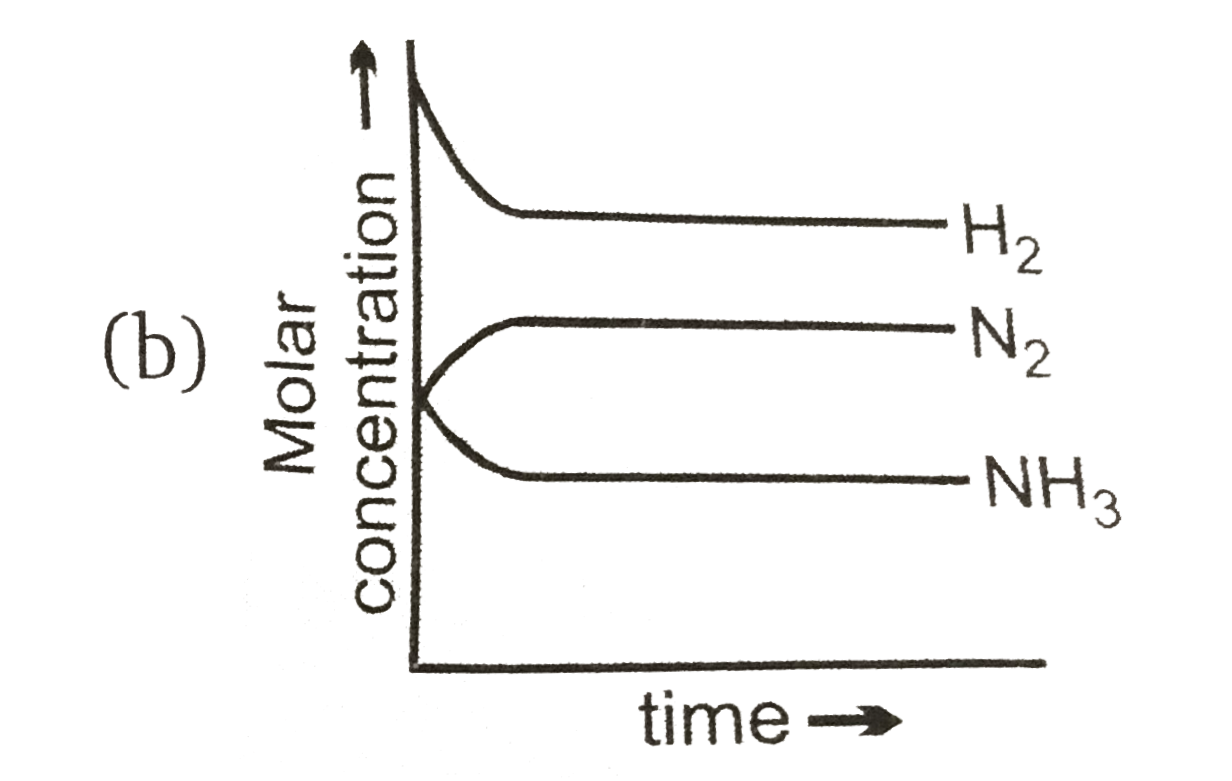
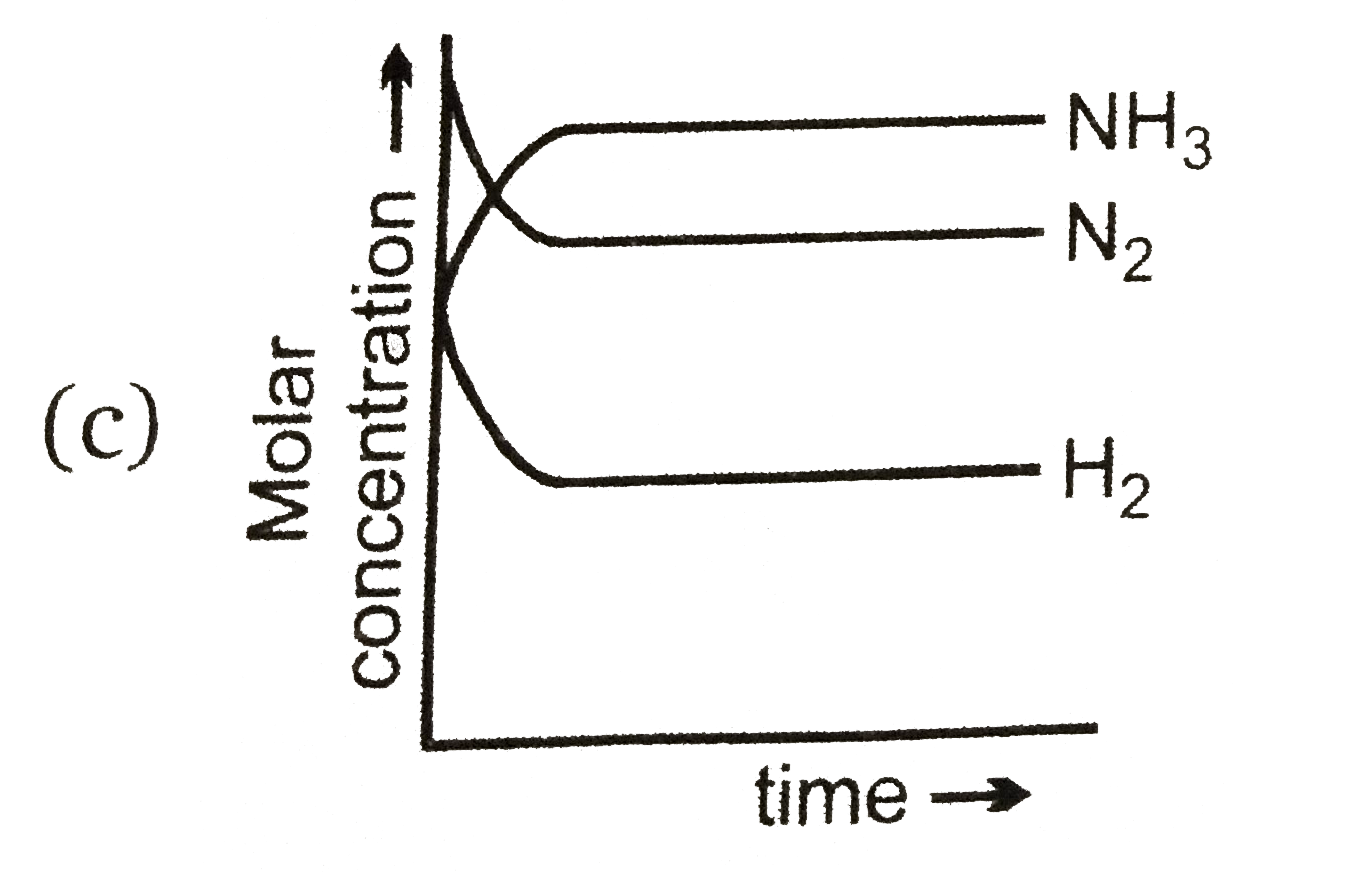
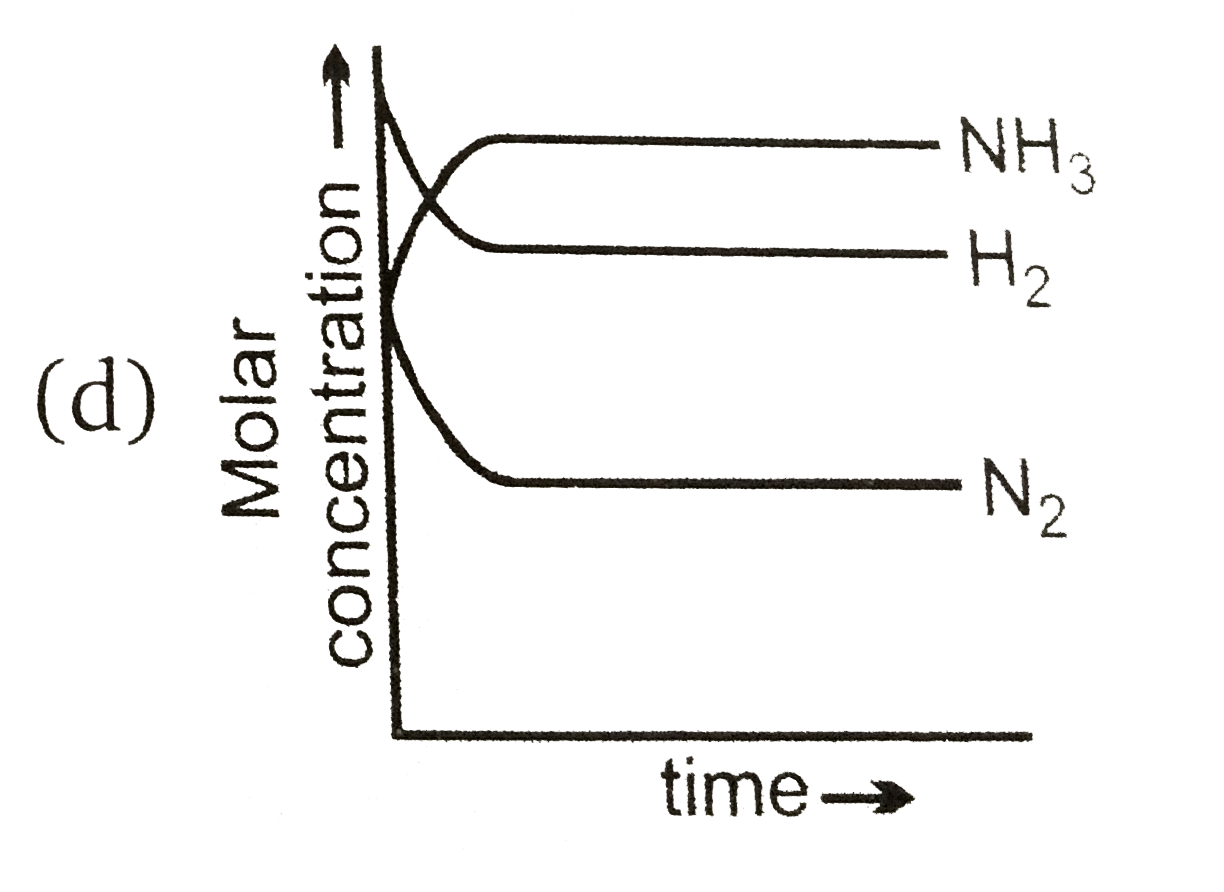
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr2NH(3) in t...
Text Solution
|
- For the reaction, N(2)+3H(2)hArr2NH(3), in a vessel equal moles of N(2...
Text Solution
|
- N(2) + 3H(2)hArr2NH(3) If temperature of following equilibrium reactio...
Text Solution
|
- For the reaction: N(2)+3H(2)hArr2NH(3) If the initial concentratio...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr2NH(3) in t...
Text Solution
|
- N(2)(g)+3H(2)(g)overset(Fe+Mo)hArr2NH(3)(g) , Haber's process, Mo is u...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2) + 3NH(2) hArr 2NH(3)...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr hNH(3) in ...
Text Solution
|
- In the manufacture of NH(3) by Haber's process, the condition which wo...
Text Solution
|