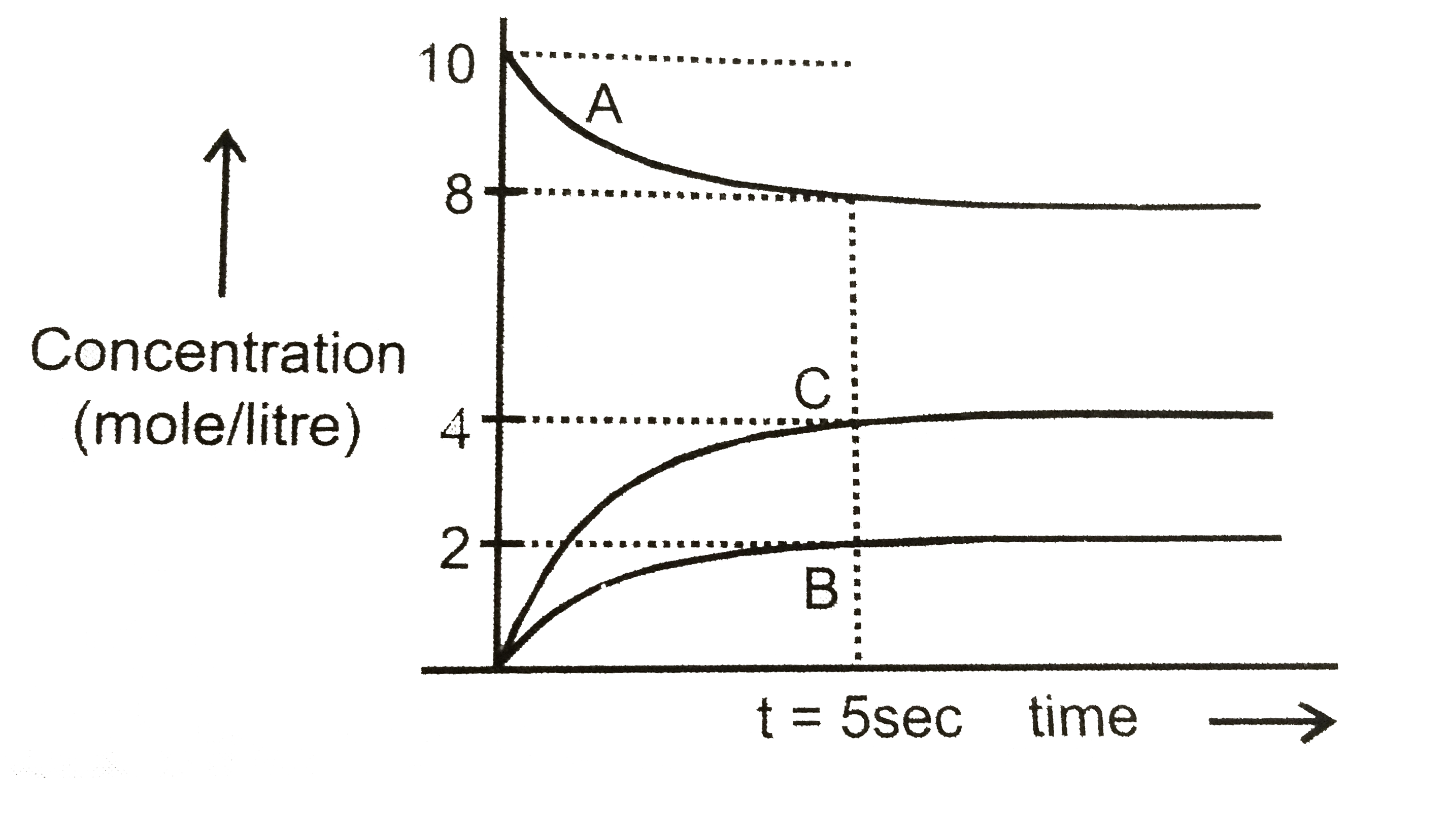
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Attainment of the equilibrium A(g)hArr2C(g)+B(g)gave the following gra...
Text Solution
|
- 2mol each of A and B are taken in a container to carry out the followi...
Text Solution
|
- In the following reaction, 3A (g)+B(g) hArr 2C(g) +D(g), Initial moles...
Text Solution
|
- Attainment of the equilibrium A(g)hArr2C(g)+B(g)gave the following gra...
Text Solution
|
- At a given temperature equilibrium is attained when 50% of each reacta...
Text Solution
|
- In the reaction AB(g) hArr A(g) + B(g) at 30^(@)C, k(p) for the dissoc...
Text Solution
|
- The equilibrium A(g) +4B(g) hArr AB4(g) is attained by mixing equal mo...
Text Solution
|
- Attainment of the equilibrium A(g)hArr2C(g)+B(g) gave the following gr...
Text Solution
|
- Kp for the reaction N2O4(g) leftrightarrow 2NO2(g) at 750K is 640torr....
Text Solution
|