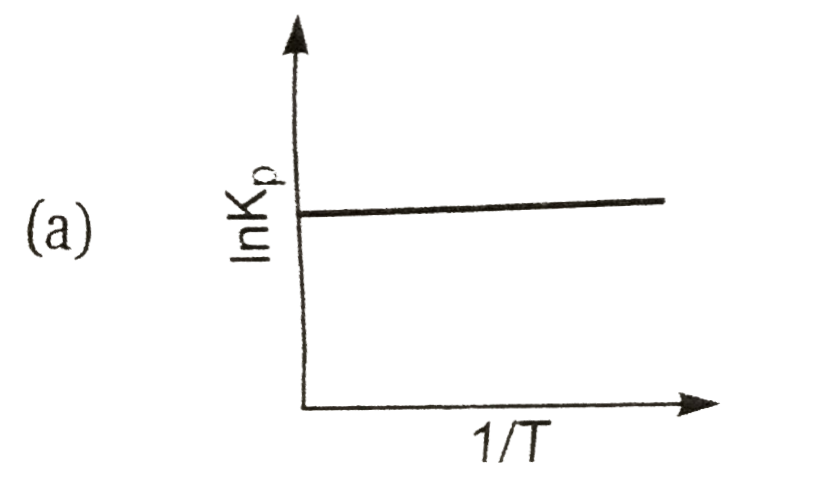
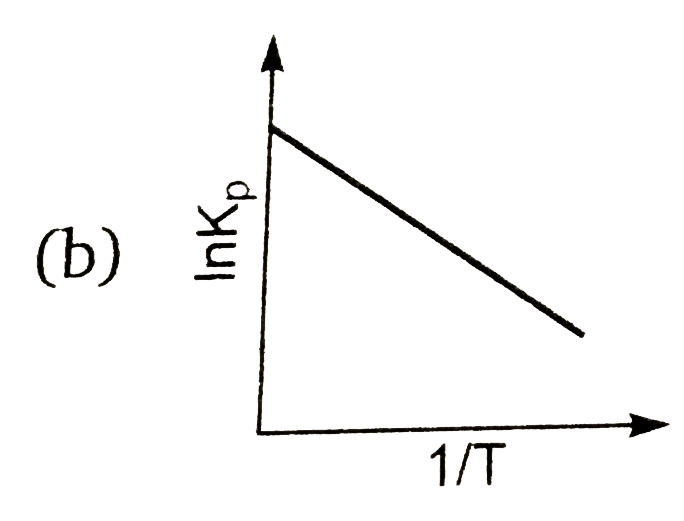
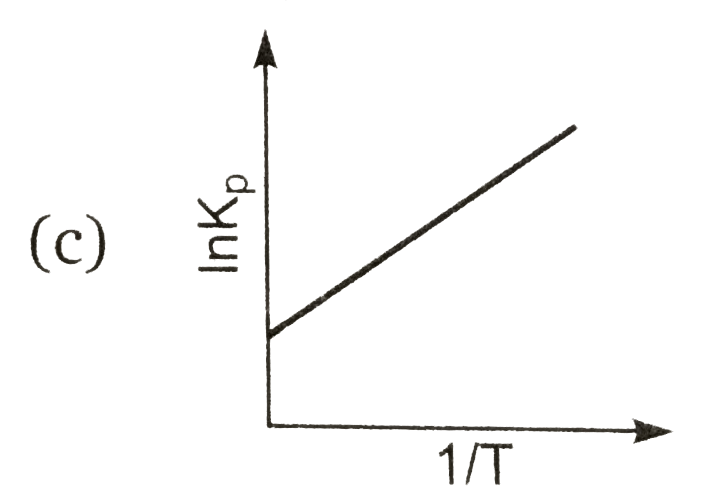
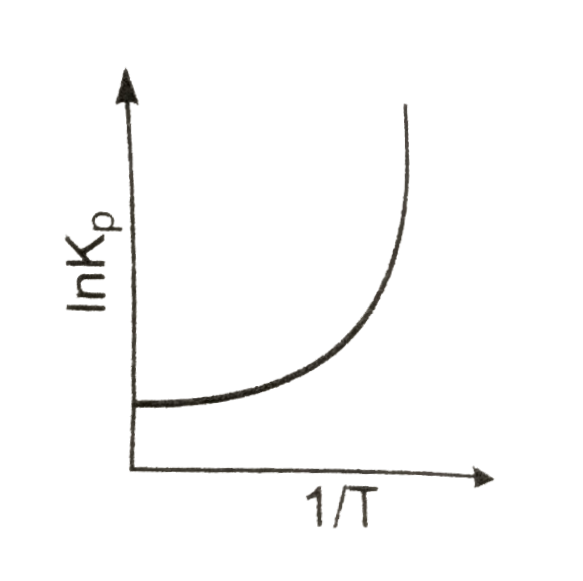
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An endothermic reaction is represented by the graph :
Text Solution
|
- An endothermic reaction is represented by the graph :
Text Solution
|
- ऊष्माशोषी तथा उष्माशेषी अभिक्रियाओं के लिए DeltaH के मान के चिन्ह क्य...
Text Solution
|
- Exothermic reaction and Endothermic reaction :
Text Solution
|
- Exothermic reaction and Endothermic reaction
Text Solution
|
- Of the reactions stated, the endothermic reaction is
Text Solution
|
- In which of the following plots, an endothermic reaction if correctly ...
Text Solution
|
- निम्नलिखित में से ऊष्माशोषी अभिक्रिया है (i) (i) मेथेन का दहन (ii)...
Text Solution
|
- ऊष्माशोषी अभिक्रिया में,
Text Solution
|