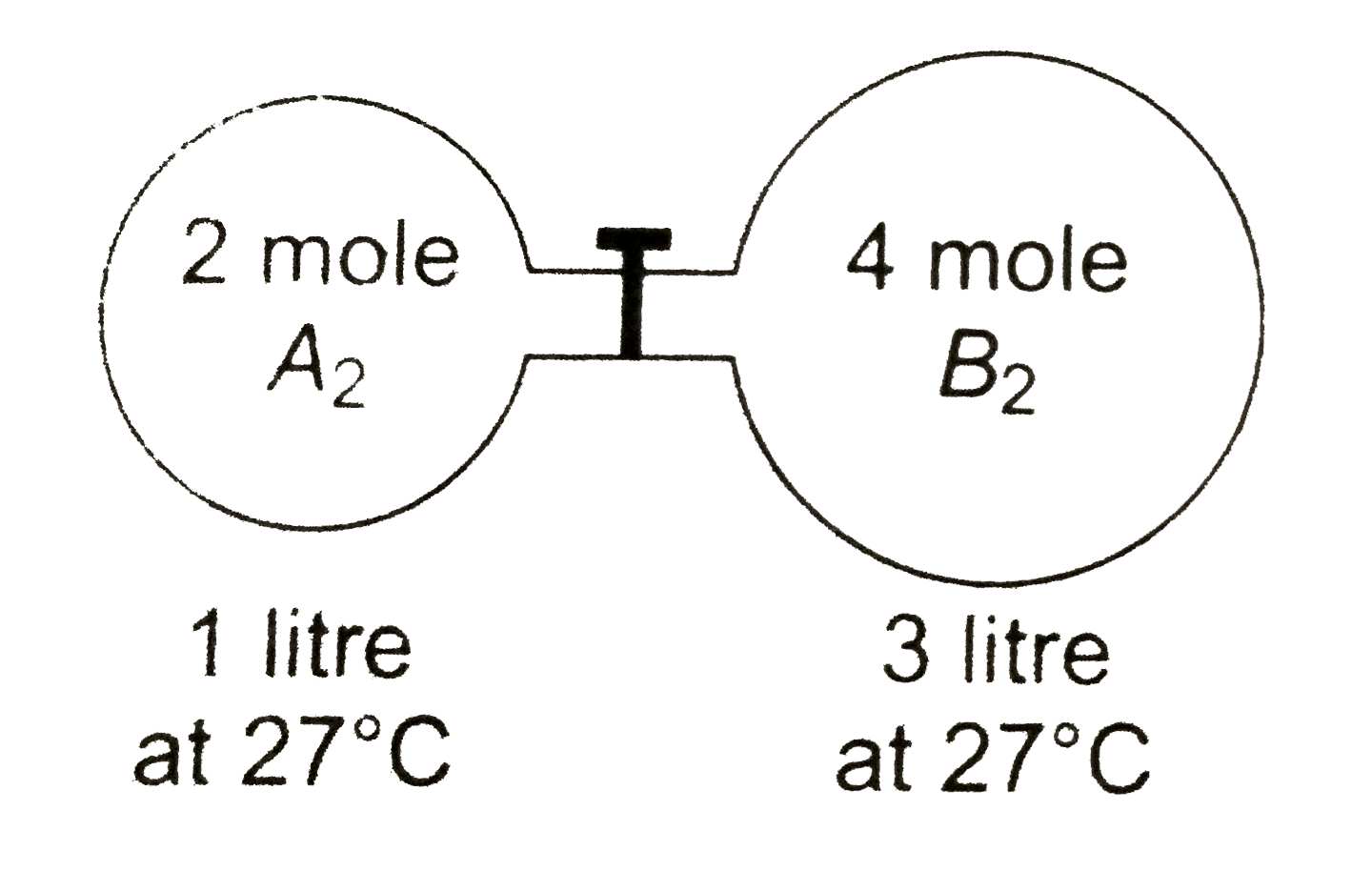A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The gas A(2) in the left flask allowed to react with gas B(2) present ...
Text Solution
|
- The value of K(c) for the reaction: A(2)(g)+B(2)(g) hArr 2AB(g) at 100...
Text Solution
|
- The equilibrium constant of the reaction A(2)(g)+B(2)(g) hArr 2AB(g) a...
Text Solution
|
- The K(c) for A(2(g))+B(2(g))hArr2AB((g)) at 100^(@)C is 50 . If one li...
Text Solution
|
- The equilibrium constant of the reaction A(2)(g)+B(2)(g)hArr2AB(g) at ...
Text Solution
|
- Determinre the value of equilibrium constant (K(C)) for the reaction ...
Text Solution
|
- The gas A(2) in the left flask allowed to react with gas B(2) present ...
Text Solution
|
- अभिक्रिया A(2)(g)+B(2)(g)hArr2AB(g) के लिए 100^(@)C पर साम्य शिरांक का...
Text Solution
|
- A(2)(g)+B(2)(g)hArr 2AB(g), equilibrium constant of the given reaction...
Text Solution
|