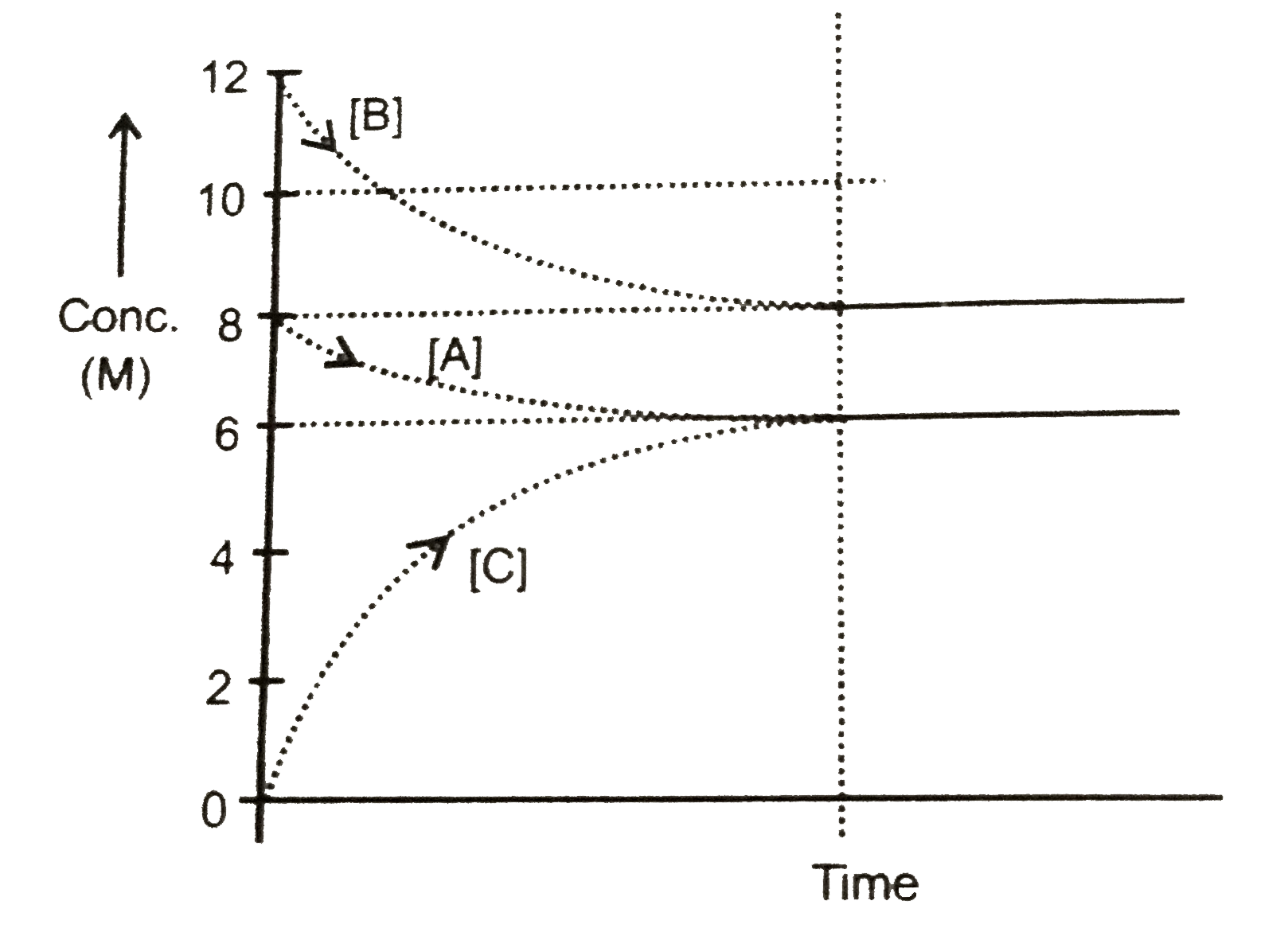
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Level 3|1 VideosCHEMICAL EQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise One more Answer is/are Correct|1 VideosCHEMICAL EQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Assertion- Reason Type Question|15 VideosATOMIC STUCTURE
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|1 VideosDILUTE SOLUTION
NARENDRA AWASTHI ENGLISH|Exercise leval-03|23 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-CHEMICAL EQUILIBRIUM-Subjective Problems
- In the reaction C(s)+CO(2)(g) hArr 2CO(g), the equilibrium pressure is...
Text Solution
|
- Calculate partial pressure of B at equilibrium in the following equili...
Text Solution
|
- In a gaseous reaction A+2B iff 2C+D the initial concentration of B was...
Text Solution
|
- For the reaction A(g) iff B (g), K(C)=10 B(g) iff C(g), K(C)=2 ...
Text Solution
|
- 5 litre vessel contains 2 moles of each of gases A and B at equilibriu...
Text Solution
|
- Calculate K(P) for the reaction A(g) iff B(s)+2C(g), K(C)=0.2 at 305 K...
Text Solution
|
- A mixture of 3 moles of SO(2), 4 moles of NO(2), 1 mole of SO(3) and...
Text Solution
|
- The density of an equilibrium mixture of N(2)O(4) and NO(2) at 1 atm a...
Text Solution
|
- If chemical equilibrium is attained at standard states then what is th...
Text Solution
|
- Calculate the equilibrium concentration ratio of C to A if equimolar r...
Text Solution
|
- A definite amount of solid NH(4)HS is placed in a flask already contai...
Text Solution
|
- The gaseous reaction : A(g)+nB(g) iff mC(g) is represented by followin...
Text Solution
|