A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-DILUTE SOLUTION-leval-03
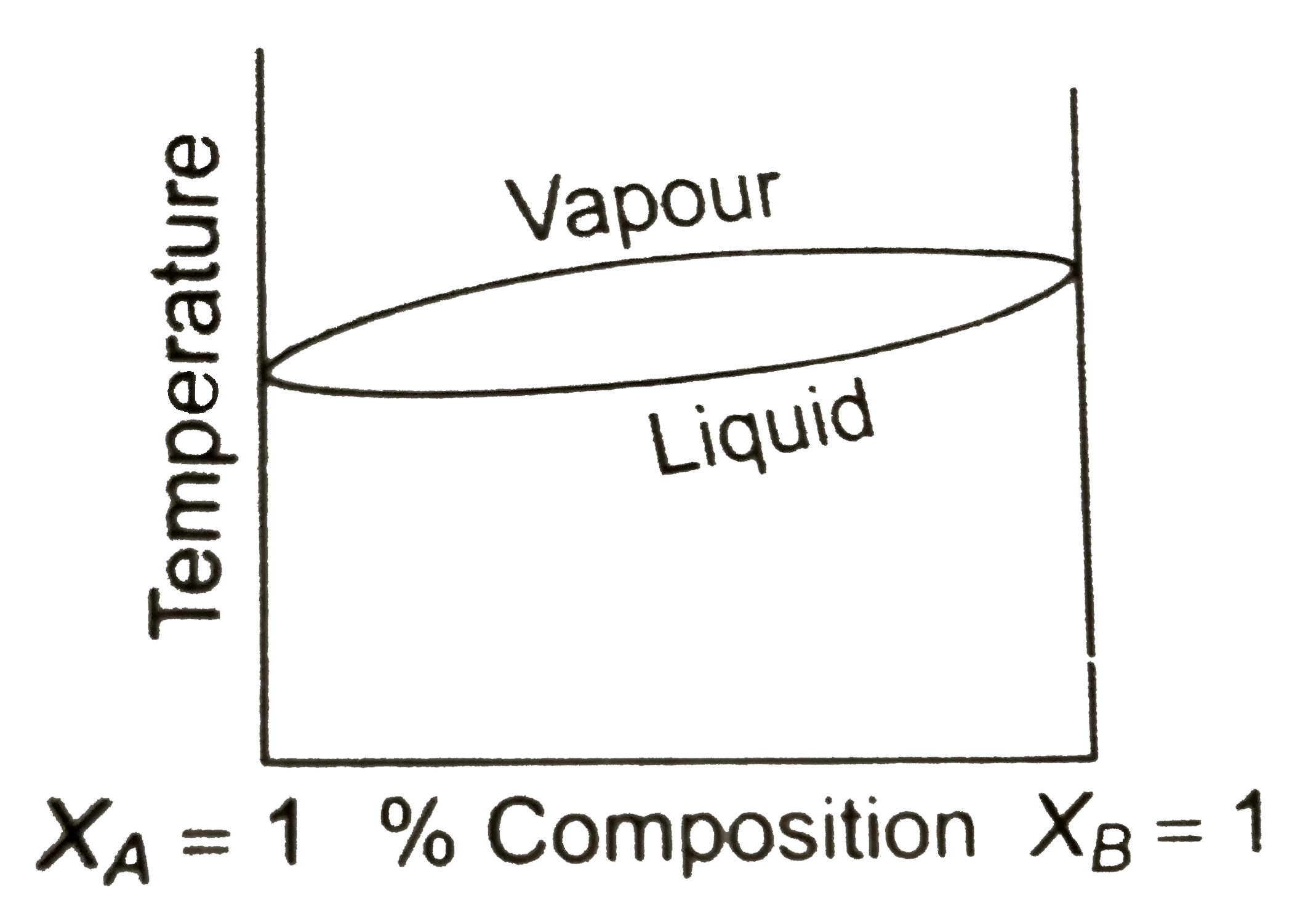
- Boiling point composition diagram of the liqid-vapour equilibrium for ...
Text Solution
|
- Lowering in vapour pressure is determined by Ostwald and Walker dynami...
Text Solution
|
- Lowering in vapour pressure is determined by Ostwald and Walker dynami...
Text Solution
|
- Lowering in vapour pressure is determined by Ostwald and Walker dynami...
Text Solution
|
- Lowering in vapour pressure is determined by Ostwald and Walker dynami...
Text Solution
|
- A dilute solution contains 'x' moles of solute A in 1 kg of solvent wi...
Text Solution
|
- A dilute solution contains 'x' moles of solute A in 1 kg of solvent wi...
Text Solution
|
- Which of the following statement(s) is/are correct, if intermolecular ...
Text Solution
|
- When non-volatile solute is added to a pure solvent, the:
Text Solution
|
- The total vapour pressure of a binary solution is gives by P = (100X(...
Text Solution
|
- Which of the following is correct for an ideal solution?
Text Solution
|
- Which of the following is correct for a non-ideal solution of liquids ...
Text Solution
|
- A binary solution of liquids A and B will show positive deviation from...
Text Solution
|
- Which of the following statement is/are correct about acetone and tric...
Text Solution
|
- The azeotropic solution of two miscible liquids:
Text Solution
|
- For exact determination of molecular mass through colligative properti...
Text Solution
|
- In the depression of freezing point experiment, it is found that the:
Text Solution
|
- The cryoscopic constant value depends upon:
Text Solution
|
- Consider 0.1 M solutions of two solutes X and Y. The solute X behaves...
Text Solution
|
- Consider following solutions: (I) I M glucose(aq) (II) 1 M so...
Text Solution
|
- Which of the following statement is (are) incorrect?
Text Solution
|