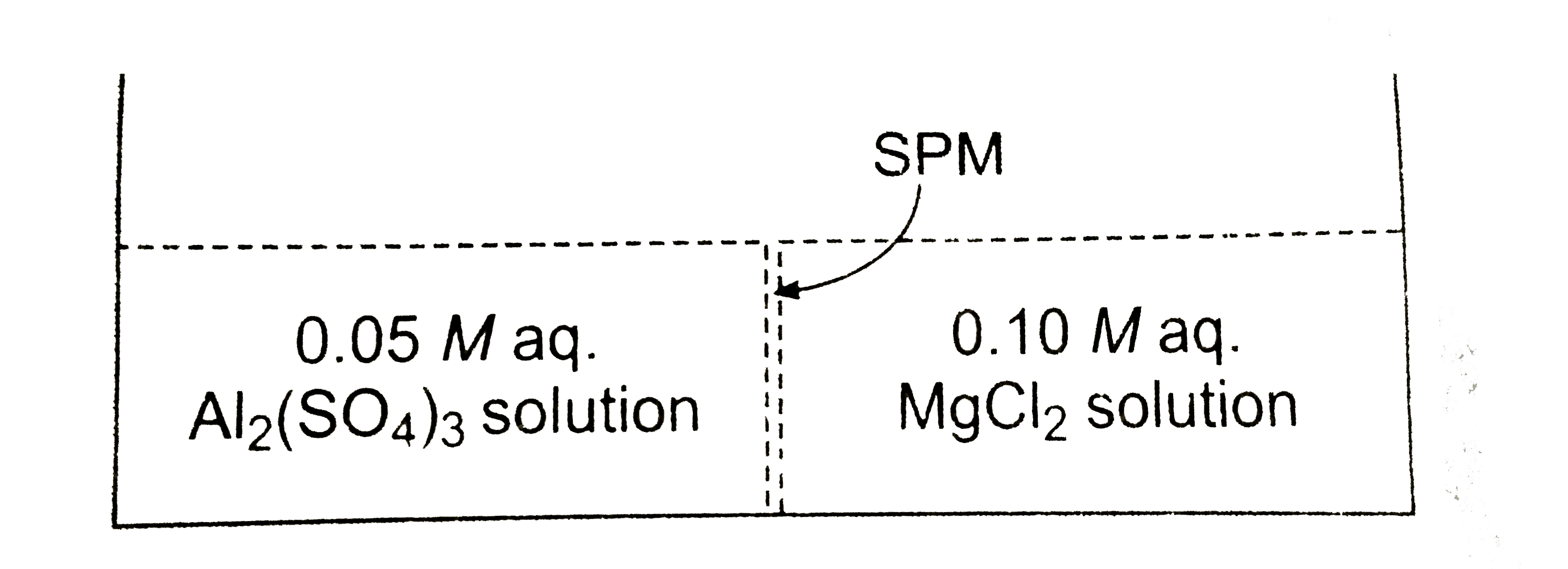A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-DILUTE SOLUTION-leval-02
- A saturated solution of XCl(3) has a vapour pressure 17.20 mm Hg at 20...
Text Solution
|
- A certain non-volatile electrolyte contain 40% carbon, 6.7% hydrogen a...
Text Solution
|
- A 0.10 M solution of a mono protic acid (d=1.01g//cm^(3)) is 5% dissoc...
Text Solution
|
- An aqueous solution boils at 101^(@)C. What is the freezing point of t...
Text Solution
|
- An industrial waste water I found to contain 8.2% Na(3)PO(4) and 12% M...
Text Solution
|
- Ratio of (/\T(b))/(K(b)) of 10 g AB(2) and 14 g A(2)B per 100 g of sol...
Text Solution
|
- The freezing point of solution containing 0.2 g of acetic acid in 20.0...
Text Solution
|
- If the boiling point of an aqueous solution containing a non-volatile ...
Text Solution
|
- 100 g of C(6)H(12)O(6) (aq.) solution has vapour pressure is equal to ...
Text Solution
|
- 1.0 g of a monobassic acid HA in 100 g water lowers the freezing poin...
Text Solution
|
- 0.1 M KI and 0.2 M AgNO(3) are mixed in 3 : 1 volume ratio. The depres...
Text Solution
|
- If 0.1 M H(2)SO(4)(aq.) solution shows freezing point -0.3906^(@)C the...
Text Solution
|
- A living cell contains a solution which is isotonic with 0.2 M glucose...
Text Solution
|
- What is the osmotic pressure of 0.2 M HX (aq.) solution at 300 K ? (a...
Text Solution
|
- A solution contain 8 g of a carbohydrate in 100 g of water has a densi...
Text Solution
|
- Study the following figure and choose the correct options. Assuming co...
Text Solution
|
- The total vapour pressure of a 4 mole % solution of NH(3) in water at...
Text Solution
|
- The vapoure pressure of two pure liquids A and B which from an ideal s...
Text Solution
|
- The composition of vapour when first bubble formed is:
Text Solution
|
- What is the composition of last droplet of liquid remaining in equilib...
Text Solution
|