In a saturated solution of AgCl, NaCl is added gradually. The concentration of `Ag^(+)` is plotted against the concentration of `Cl^(-)`. The graph appears as :
In a saturated solution of AgCl, NaCl is added gradually. The concentration of `Ag^(+)` is plotted against the concentration of `Cl^(-)`. The graph appears as :
A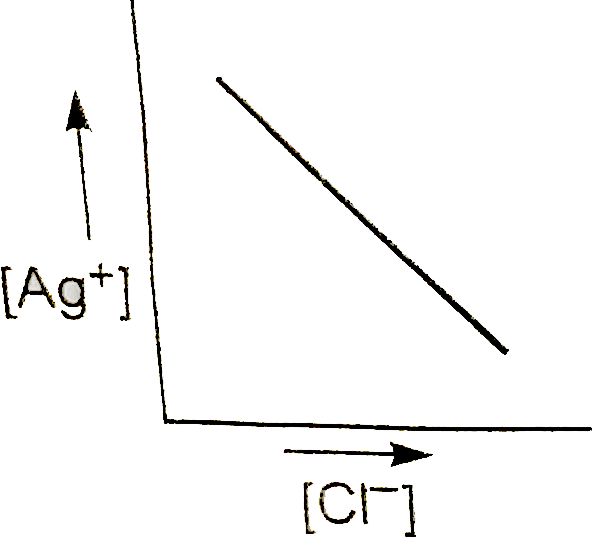

B

C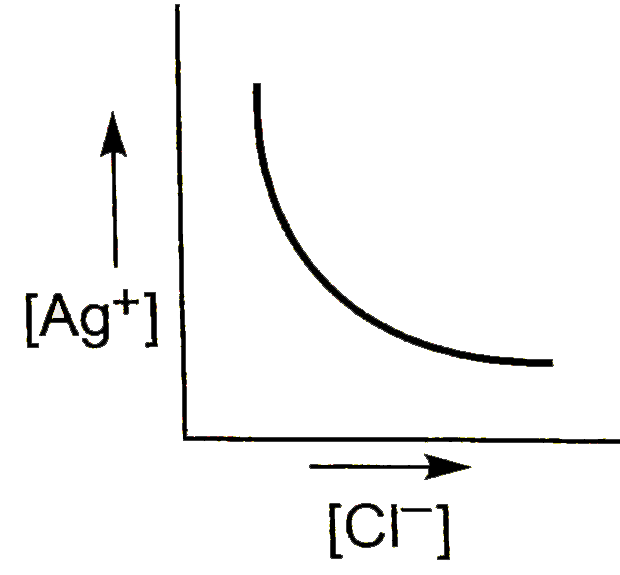

D

Text Solution
AI Generated Solution
The correct Answer is:
To solve the problem of how the concentration of \( \text{Ag}^+ \) changes as \( \text{NaCl} \) is added to a saturated solution of \( \text{AgCl} \), we can follow these steps:
### Step 1: Understand the Dissociation of \( \text{AgCl} \)
The dissociation of silver chloride (\( \text{AgCl} \)) in water can be represented as:
\[
\text{AgCl (s)} \rightleftharpoons \text{Ag}^+ (aq) + \text{Cl}^- (aq)
\]
In a saturated solution of \( \text{AgCl} \), the concentrations of \( \text{Ag}^+ \) and \( \text{Cl}^- \) are in equilibrium.
### Step 2: Adding \( \text{NaCl} \)
When \( \text{NaCl} \) is added to the solution, it dissociates completely into \( \text{Na}^+ \) and \( \text{Cl}^- \):
\[
\text{NaCl (s)} \rightleftharpoons \text{Na}^+ (aq) + \text{Cl}^- (aq)
\]
This increases the concentration of \( \text{Cl}^- \) ions in the solution.
### Step 3: Apply the Common Ion Effect
The increase in \( \text{Cl}^- \) concentration due to the addition of \( \text{NaCl} \) will shift the equilibrium of the \( \text{AgCl} \) dissociation reaction to the left, according to Le Chatelier's principle. This results in a decrease in the concentration of \( \text{Ag}^+ \):
\[
\text{Ag}^+ + \text{Cl}^- \rightleftharpoons \text{AgCl (s)}
\]
Thus, as \( [\text{Cl}^-] \) increases, \( [\text{Ag}^+] \) decreases.
### Step 4: Relationship Between Concentrations
The solubility product constant (\( K_{sp} \)) for \( \text{AgCl} \) is given by:
\[
K_{sp} = [\text{Ag}^+][\text{Cl}^-]
\]
If we let \( s \) be the solubility of \( \text{AgCl} \), then:
\[
K_{sp} = s^2
\]
As \( [\text{Cl}^-] \) increases (due to the addition of \( \text{NaCl} \)), \( [\text{Ag}^+] \) must decrease to maintain the constant \( K_{sp} \).
### Step 5: Graphical Representation
When plotting \( [\text{Ag}^+] \) against \( [\text{Cl}^-] \):
- Initially, as \( [\text{Cl}^-] \) increases, \( [\text{Ag}^+] \) decreases.
- The graph will show a downward trend, which can be represented as a curve that approaches the x-axis but does not touch it, indicating that \( [\text{Ag}^+] \) approaches zero as \( [\text{Cl}^-] \) increases.
### Conclusion
The graph of \( [\text{Ag}^+] \) versus \( [\text{Cl}^-] \) will appear as a downward curve, indicating the inverse relationship due to the common ion effect.
---
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
How much the concentration of Ag^(o+) ions in a saturated solution of AgCI diminish if such an amount of HCI is added to it that the concentration of CI^(Θ) ions in the solution becomes equal to 0.03M ? Also find the amount of AgCI precipitated at the given concentration. K_(sp) of AgCI = 1.8 xx 10^(-10) .
NaCl solution is added to a saturated solution of PbCl_(2). What will happen to the concentration of Pb^(+2) ions?
NaCI solution is added to a saturated solution of PbCI_2 . What will happen to the concentration of Pb^(+2) ions ?
The shape of the curve obtained when substrate concentration is plotted against velocity of reaction
In a saturated aqueous solution of AgBr, concentration of Ag^(+) ion is 1xx10^(-6) mol L^(-1) it K_(sp) for AgBr is 4xx10^(-13) , then concentration of Br^(-) in solution is
If a thin slice of sugar beet is placed in concentrated solution of NaCl , then
In 0.1M HCl solution, 0.1M NaOH solution is added gradually then identify the correct graph in this titration
The graph plotted between concentration versus time
The product of the concentration of the ions of an electrolyte raised to power of their coefficients in the balanced chemical equation in the solution at any concentration . Its value is not constant and varies with change in concentration . Its value is not constant and varies with change in concentration . Its value is not constant and varies with change in concentration . Ionic product of the saturated solution is called solubility product K_(sp) (i) When K_(ip) = K_(sp) , the solution is just saturated and no precipitation takes place . (ii) When K_(ip) lt K_(sp) the solution is unsaturated and precipitation will not take place . When K_(ip) gt K_(sP) the solution is supersaturated and precipitation takes place . The concentration of Ag^(+) ions in a given saturated solution of AgCl at 25^(@)C is 1.06 xx10^(-5) g ion per litre . The solubility product of AgCl is :
The solubility proudct of AgCl at 25^(@)C is 1xx10^(-10) A solution of Ag^(+) at a concentration 4xx10^(-3) M just fails to yield a prenciitate of AgCl with concentration of 1xx10^(-3) M Cl^(-) when the concentration of NH_(3) in the solution is 2xx10^(-2)M. Calculate the equlibrium constant for [Ag(NH_(3))_(2))hArrAg^(+)+2NH_(3)