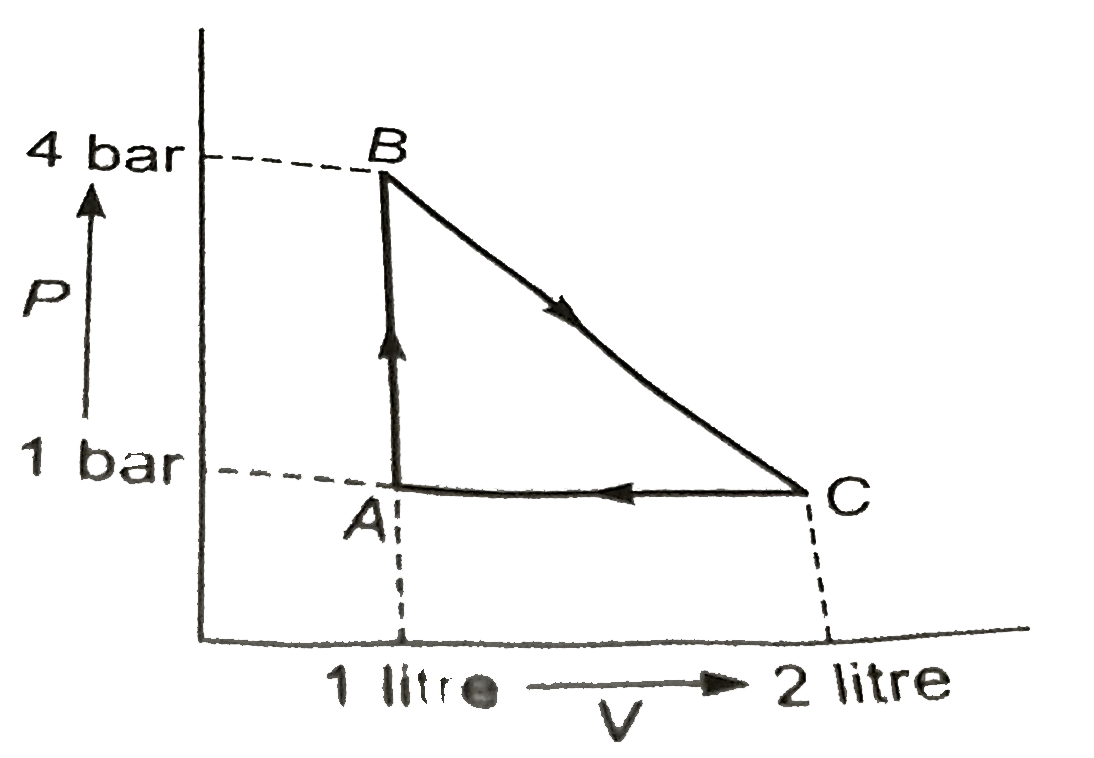
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-THERMODYNAMICS-Level 2
- 1 mole of an ideal gas A(C(v.m)=3R) and 2 mole of an ideal gas B are...
Text Solution
|
- Calculate the work done by the system in an irreversible (single step)...
Text Solution
|
- One mole of an ideal gas is carried through the reversible cyclic proc...
Text Solution
|
- Two moles of a triatomic linear gas (neglect vibration degree of freed...
Text Solution
|
- A gas (C(v.m) = (5)/(2)R) behaving ideally is allowed to expand revers...
Text Solution
|
- Two mole of an ideal gas is heated at constant pressure of one atmosp...
Text Solution
|
- 10 mole of an ideal gas is heated at constant pressure of one atmosphe...
Text Solution
|
- For polytropic process PV^(n) = constant, molar heat capacity (C(m)) o...
Text Solution
|
- 2 mole of an ideal monoatomic gas undergoes a reversible process for w...
Text Solution
|
- Calculate DeltaS for 3 mole of a diatomic ideal gas which is heated an...
Text Solution
|
- One mole of an ideal monoatomic gas at 27^(@)C is subjected to a rever...
Text Solution
|
- Two moles of an ideal gas is expanded irreversibly and isothermally at...
Text Solution
|
- For a perfectly crystalline solid C(p,m)=aT^(3)+bT, where a and b are ...
Text Solution
|
- Which of the following statement (s) is correct? Statement-I: The en...
Text Solution
|
- Combustion of sucrose is used by aerobic organisms for providing energ...
Text Solution
|
- For the hypothetical reaction A(2)(g)+B(2)(g) to 2AB(g) DeltaG(r)^...
Text Solution
|
- Calculate Delta(r)G^(@) for (NH(4)Cl,s) at 310K. Given :Delta(r)H^(@...
Text Solution
|
- Using listed informations, calculate Delta(r)G^(@) (in kJ/mol) at 27^(...
Text Solution
|
- Fixed mass of an ideal gas collected in a 24.64 litre sealed rigid ves...
Text Solution
|
- The molar heat capacities at constant pressure (assumed constant with ...
Text Solution
|