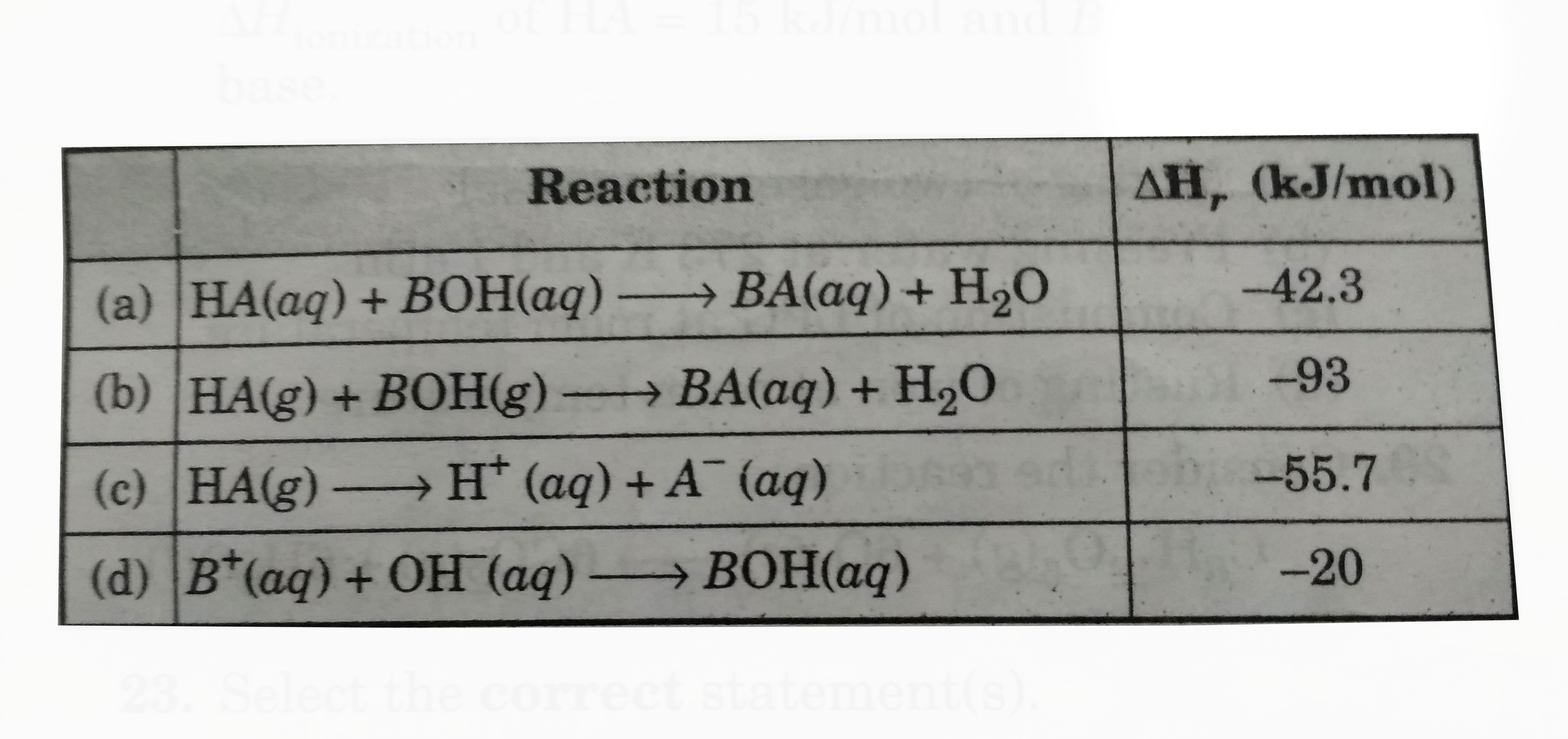
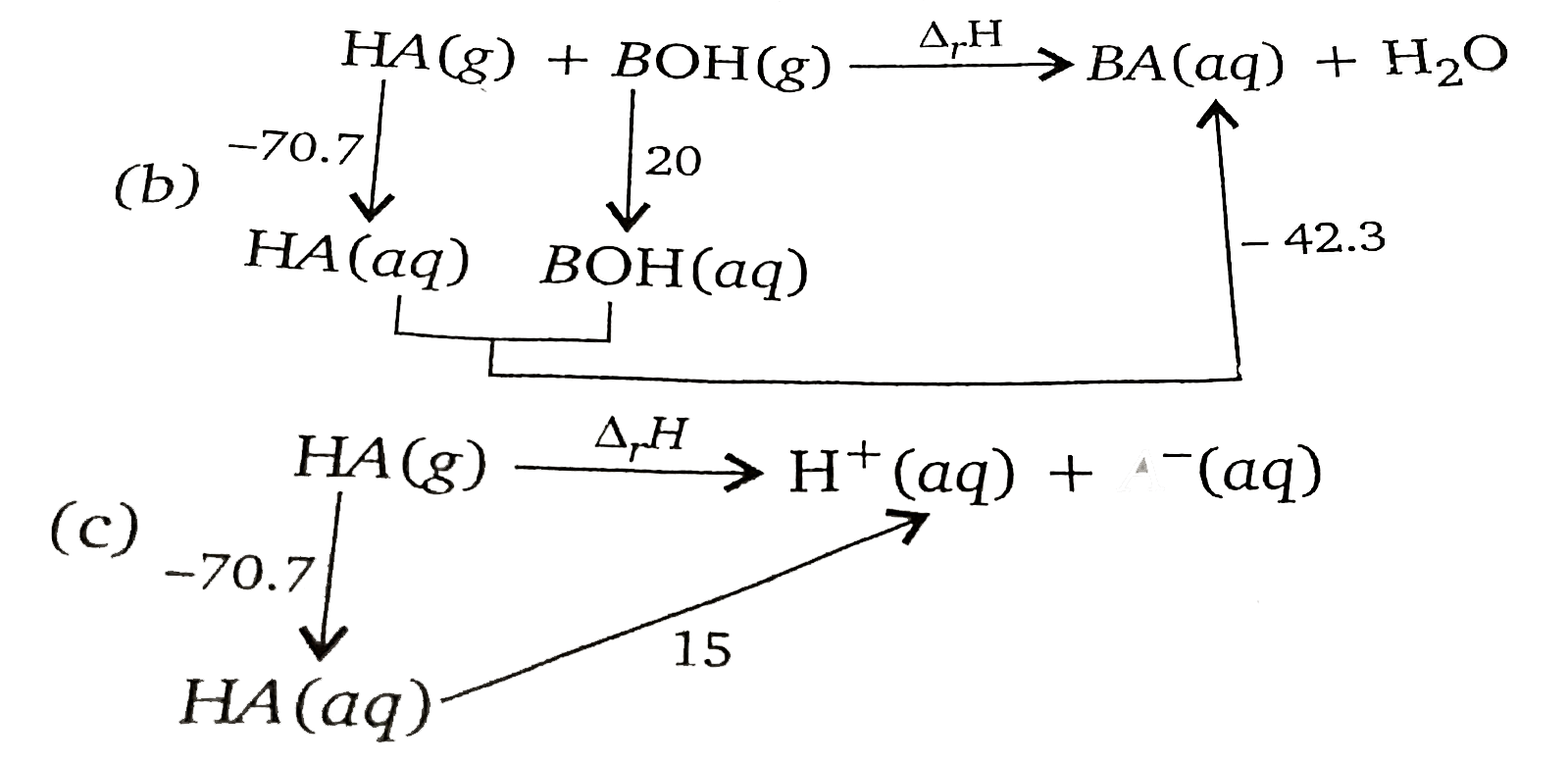
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-THERMODYNAMICS-Level 3
- Which of the following statement(s) is/are false?
Text Solution
|
- Which of the following statement(s) is/are true?
Text Solution
|
- From the following date , mark the opation(s) where Delta H is correct...
Text Solution
|
- Select correct statement(s)
Text Solution
|
- For an isolated system, the entropy :
Text Solution
|
- The normal boiling point of a liquid X is 400 K. DeltaH("vap") at norm...
Text Solution
|
- Select incorrect statement(s)
Text Solution
|
- Select correct statement(s) for the reaction H(2)O(g)+CO(g)rarrH(2)(g)...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following Column - I and Column - II
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|