Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-THERMODYNAMICS-Level 3
- Statment 1|Delta(f)H|" of "(H(2)O,l)gt|Delta(f)H|" of "(H(2)O,g) S...
Text Solution
|
- All combustion reactions are exothermic. Enthalpies of products are ...
Text Solution
|
- Enthalpy of neutralization of CH(3)COOH by NaOH is less than that of H...
Text Solution
|
- Assertion:-Internal energy of a real gas may change during expansion a...
Text Solution
|
- Work is a state function which is expressed in joule. work appears ...
Text Solution
|
- The expansion of a gas into an evacuated space takes place non-spontan...
Text Solution
|
- A perfect gas undergoes a reversible adiabatic expansion from (300 K, ...
Text Solution
|
- 5 mole of an ideal gas at temp. T are compressed isothermally from 12 ...
Text Solution
|
- A diatomic ideal gas is expanded according to PV^(3) = constant, under...
Text Solution
|
- A heat engine is operating between 500 K to 300 K and it absorbs 10 kc...
Text Solution
|
- Molar heat capacities at constant pressure for A, B and C are 3, 1.5 a...
Text Solution
|
- Standard molar enthalpy of combustion of glucose is -2880 kJ. If only ...
Text Solution
|
- Given C(2)H(2)(g)+H(2)(g)rarrC(2)H(4)(g): DeltaH^(@)=-175 " kJ mol"^(-...
Text Solution
|
- The integral enthalpies of solution of anhydrous CuSO(4) (s) and hydra...
Text Solution
|
- If enthalpy of neutralisation of HCl by NaOH is -57 kJ " mol"^(-1) and...
Text Solution
|
- Lattice energy of NaCl(s) is -790 kJ " mol"^(-1) and enthalpy of hydra...
Text Solution
|
- x g sample of NH(4)NO(3) is decomposed in a Bomb calorimeter. The temp...
Text Solution
|
- A heat engine operating between 227^(@)C and 77^(@)C absorbs 10 kcal o...
Text Solution
|
- Calculate work done in chemical reaction (in kcal) A(s)+3B(g)rarrC(l...
Text Solution
|
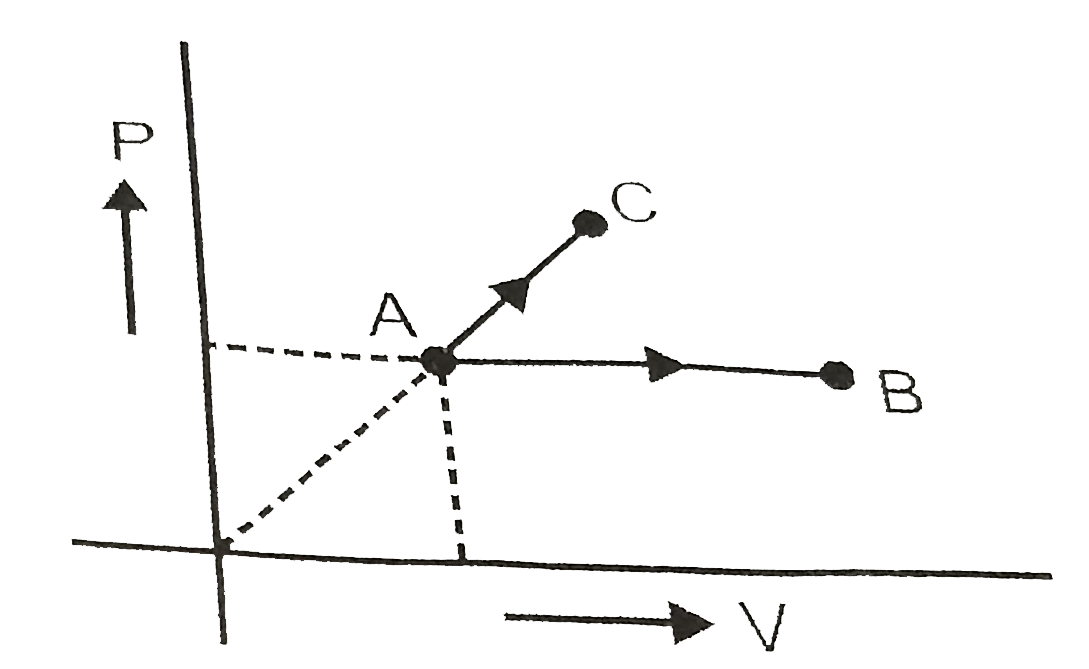
- One mole ideal monoatomic gas is heated according to path AB and AC. ...
Text Solution
|