A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Level 3 Passage 1|4 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Level 3 Passage 2|6 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|15 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|14 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-GASEOUS STATE-Level 2
- Three closed rigid vessels, A, B and C, which initially contain three ...
Text Solution
|
- Gas molecules each of mass 10^(-26) kg are taken in a container of vol...
Text Solution
|
- A balloon of diameter 21 meter weight 100 kg. Calculate its pay-load, ...
Text Solution
|
- A given volume of ozonised oxygen (containing 60% oxygen by volume ) r...
Text Solution
|
- If 250 mL of N(2) over water at 30^(@)C and a total pressure of 740 t...
Text Solution
|
- A bulb of constant volume is attached to a manometer tube open at othe...
Text Solution
|
- A mixture of nitrogen and water vapours is admitted to a flask at 760 ...
Text Solution
|
- At room temperature following traction goes to completion 2AB(g)+B(2...
Text Solution
|
- A vessel of uniform cross-section of length 500 cm as shown in figure ...
Text Solution
|
- For a real gas (mol.mass =60) if density at critical point is 0.80g//c...
Text Solution
|
- The van der Waals' constant 'b' of a gas is 4pixx 10^(-4)L//mol. How n...
Text Solution
|
- The density of vapour of a substance (X) at 1 atm pressure and 500 K ...
Text Solution
|
- van der Waal's gas equation can be reduced to virial eqation and viria...
Text Solution
|
- If the slope of 'Z' (compressibility factor) vs. 'p' curve is constant...
Text Solution
|
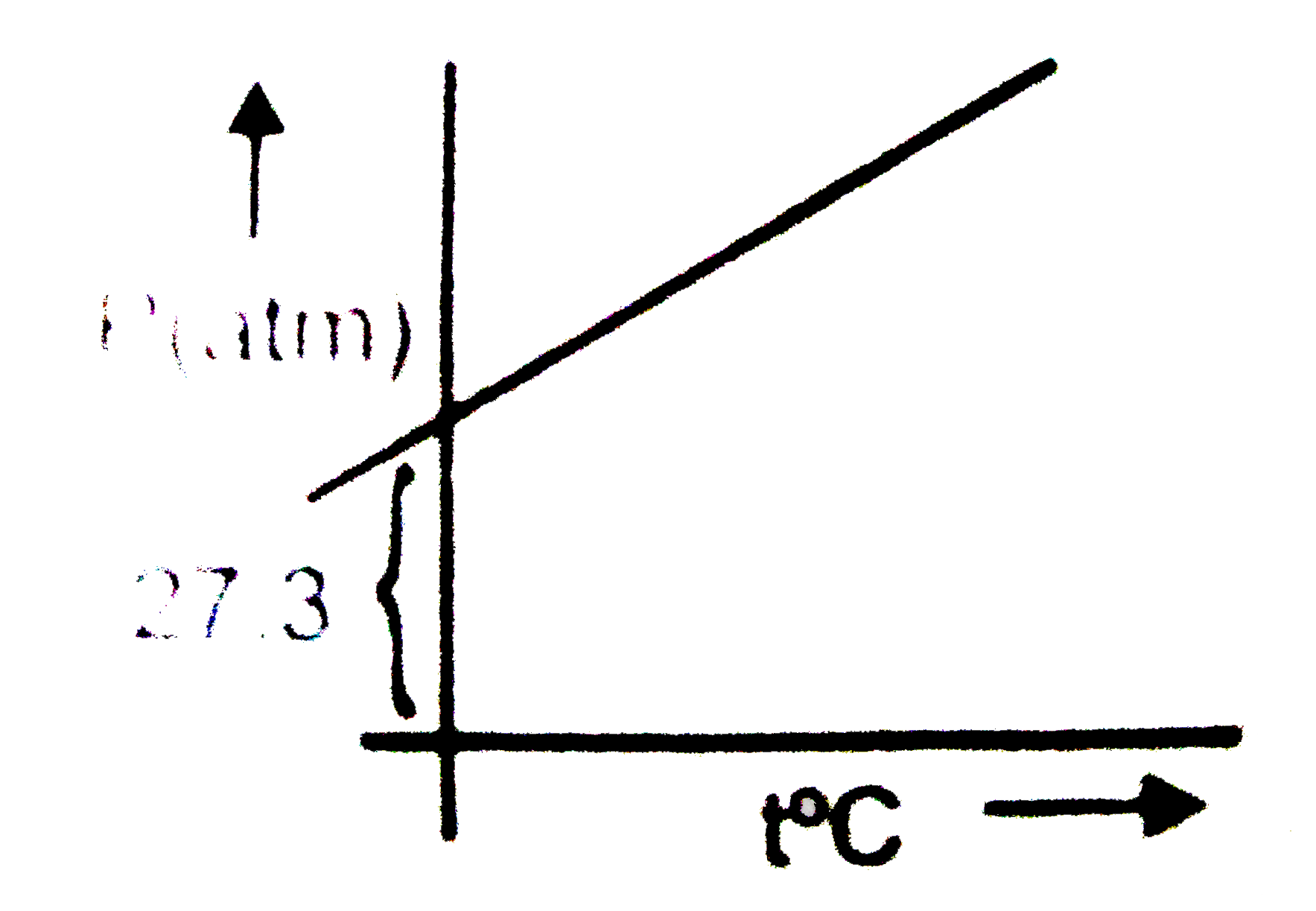
- A graph is plotted between p (atm) vs t^(@)C for 10 mol of an ideal ga...
Text Solution
|
- For two samples of ideal gases A and B curves are plotted n vs V (volu...
Text Solution
|
- At a constant temperature what should be the percentage increase in pr...
Text Solution
|
- 6 litre H(2)O is placed in a closed evacuated room of volume 8.27 litr...
Text Solution
|
- Match the items of colums I and II.
Text Solution
|
- Match the following columns
Text Solution
|