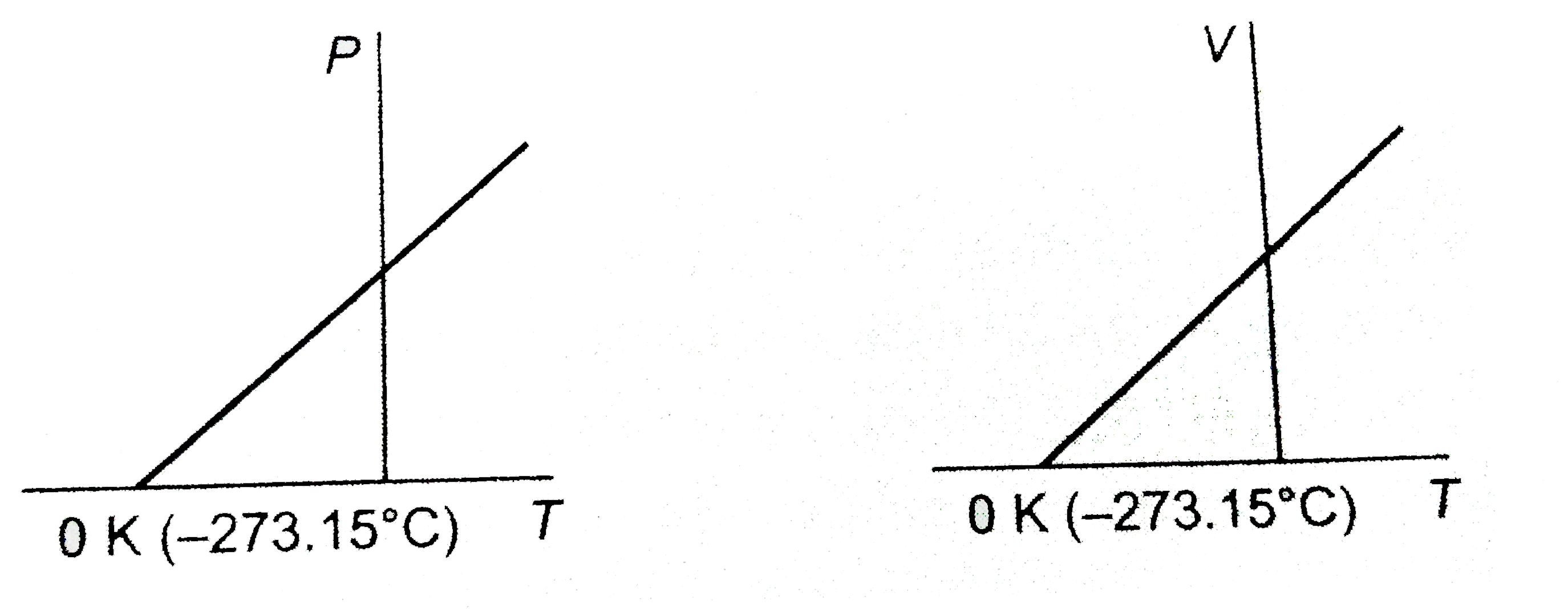
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Match the Column|7 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Assertion-ReasonType Questions|16 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Level 3 Passage 3|4 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|14 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-GASEOUS STATE-One or More Answers is/are Correct
- Which of the following curves represent(s) Boyle's law?
Text Solution
|
- If a gas expands at constant temperature
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- What conclusion would you draw from the following graphs for an ideal ...
Text Solution
|
- Which of the following is a character of a gas at Boyle temperature?
Text Solution
|
- Indicate the correct statement for equal volumes of N(2)(g) and CO(2)(...
Text Solution
|
- Which of the following is correct for critical temperature ?
Text Solution
|
- Consider the following statement regarding Maxwell's distribution of s...
Text Solution
|
- If a gas espands at a constant pressure by providing heat :
Text Solution
|
- Select the incorrect statement(s) :
Text Solution
|
- Following represents the Maxwell distribution curve for an ideal gas a...
Text Solution
|
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- Select the correct statement(s) :
Text Solution
|
- Select the correct statement :
Text Solution
|
- A open ended mercury manometer is used to measure the pressure exerte...
Text Solution
|
- Select incorrect statement for real gases :
Text Solution
|
- Select correct statements:
Text Solution
|
- Which is/are correct for real gases?
Text Solution
|
- Select incorrect statement (s)
Text Solution
|
- If an ideal gas is heated at constant pressure :
Text Solution
|