Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-GASEOUS STATE-Subjective problems
- A bubble of gas released at the bottom of a lake increases to four t...
Text Solution
|
- A gaseous mixture containing equal mole sof H(2),O(2) and He is subjec...
Text Solution
|
- One mole of a gas changed from its initial state (15L,2 atm) to final ...
Text Solution
|
- Two moles of an ideal gas undergoes the following process. Given that ...
Text Solution
|
- 1 mole of a diatomic gas present in 10 L vessel at certain temperature...
Text Solution
|
- The graph of compressibility factor (Z) vs. P for one mole of a real g...
Text Solution
|
- Under the identical conditions of temperature, the density of a gas X...
Text Solution
|
- The time for a certain volume of a gas A to diffuse through a small ho...
Text Solution
|
- Excess F(2)(g) reacts at 150^(@)C and 1.0 atm pressure with Br(2)(g) t...
Text Solution
|
- Initially bulb "a" contained oxygen gas at 27^(@)C and 950 mm of Hg an...
Text Solution
|
- Air is trapped in a horizontal glass tube by 36 cm mercury column as s...
Text Solution
|
- A flask containing air at 107^(@)C and 722 mm of Hg is cooled to 100 K...
Text Solution
|
- If an ideal gas at 100 K is heated to 109 K in a rigid container, the ...
Text Solution
|
- The van der Waals' constantes for a gas are a=3.6 atmL^(2)mol^(-2),b=0...
Text Solution
|
- A flask has 10 molecules out of which four molecules are moving at 7 m...
Text Solution
|
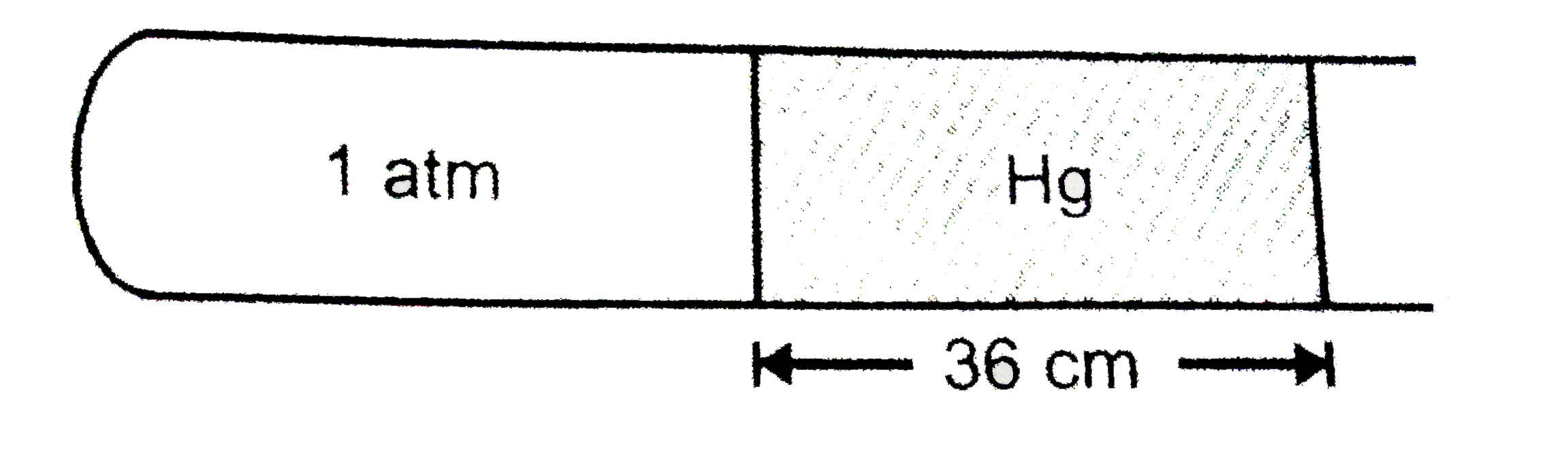 If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?
If the tube is held vertical keeping the open end up, lengh of air column shrink to 19 cm. What is the lengh (in cm) by which the mercury column shifts down?