For the solution of the gases `w,x,y and z` in water at `298 K` the Henry's law constant `(K_(H))` are `0.5,2,35` and `40 k bar`,respectively. The correct plot for the given data is :
For the solution of the gases `w,x,y and z` in water at `298 K` the Henry's law constant `(K_(H))` are `0.5,2,35` and `40 k bar`,respectively. The correct plot for the given data is :
A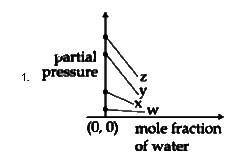

B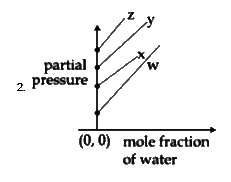

C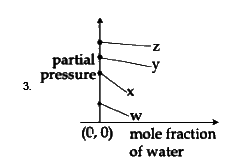

D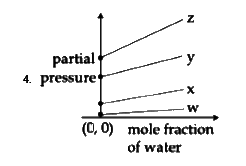

Text Solution
AI Generated Solution
The correct Answer is:
To solve the problem, we need to analyze the Henry's law constants for the gases W, X, Y, and Z and determine the correct plot based on their values. Here’s a step-by-step solution:
### Step 1: Understand Henry's Law
Henry's law states that the partial pressure of a gas in a solution is directly proportional to its mole fraction in that solution. The equation can be expressed as:
\[ P = K_H \cdot X \]
where:
- \( P \) = partial pressure of the gas
- \( K_H \) = Henry's law constant
- \( X \) = mole fraction of the gas
### Step 2: Identify the Given Data
The Henry's law constants for the gases are:
- \( K_H \) for W = 0.5 kbar
- \( K_H \) for X = 2 kbar
- \( K_H \) for Y = 35 kbar
- \( K_H \) for Z = 40 kbar
### Step 3: Determine the Relationship
From the equation \( P = K_H \cdot X \), we can rearrange it to express the mole fraction of water:
\[ P = K_H \cdot (1 - X_{water}) \]
This indicates that as the mole fraction of water increases, the partial pressure of the gas decreases, resulting in a downward slope in the plot.
### Step 4: Analyze the Slope and Intercepts
- The slope of the plot will be negative because of the inverse relationship between the mole fraction of water and the partial pressure of the gas.
- The y-intercept of the plot will be equal to the Henry's law constant \( K_H \) for each gas.
### Step 5: Compare the Values
The values of \( K_H \) indicate the y-intercepts:
- For W (0.5 kbar), the y-intercept is low.
- For X (2 kbar), the y-intercept is slightly higher.
- For Y (35 kbar), the y-intercept is much higher.
- For Z (40 kbar), the y-intercept is the highest.
### Step 6: Determine the Correct Plot
We need to find a plot that:
1. Has a downward slope (indicating a negative relationship).
2. Shows the correct order of y-intercepts based on the values of \( K_H \):
- W < X < Y < Z
### Step 7: Evaluate the Options
- The correct plot will show W closest to the origin, followed by X, then Y, and finally Z, all in a downward sloping manner.
### Conclusion
Based on the analysis, the correct plot corresponds to the first option, which accurately reflects the values of \( K_H \) and the negative slope as per Henry's law.
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
For the solution of the gases W, X, Y and Z in water at 298 K , the Henry's law constants ( K _ H ) are 0.5, 2, 35 and 40 kbar, respectively. The correct plot for the given data is :
For the solution of the gases W, X, Y and z in water at 298 K , the Henrys law constants ( K _ H ) are 0.5, 2, 35 and 40 kbar, respectively. The correct plot for the given data is :
The air is a mixture of a number of gases. The maojr components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if the Henry's law constants for oxygen and nitrogen at 298 K are 3.30xx10^(7) mm and 6.51xx10^(7) mm respectively, calculate the composition of these gases in water.
Let gas (A) present in air is dissolved in 20 moles of water at 298K and 20 atm pressure. The mole fraction of gas (A) in air is 0.2 and the Henry's law constant for solubility of gas (A) in water at 298K is 1×10^5atm.The number of mole of gas (A) dissolved in water will be
When a gas is bubbled through water at 298 K, a very dilute solution of gas is obtained . Henry's law constant for the gas is 100 kbar. If gas exerts a pressure of 1 bar, the number of moles of gas dissolved in 1 litre of water is
For a solution of acetone in chloroform, Henry's law constant is 150 torr at a temperature of 300 K. (a) Calculate the vapour pressure of acetone when the mole fraction is 0.12. (b) Assuming that Henry's law is applicable over sufficient range of composition to make the calculation valid, calculate the composition at which Henry's law pressure of chloroform is equal to Henry's law pressure of acetone at 300 K. (Henry's law constant for chloroform is 175 torr.)
1 litre of water under a nitrogen pressure of 1 bar dissolves 2xx10^(-5) kg of nitrogen at 293 K. Calculate Henry's law constant.
Henry's law constant for oxygen and nitrogen dissolved in water at 298 K are 2.0 xx 10^(9) Pa and 5.0 xx 10^(9) Pa , respectively . A sample of water at a temperature just above 273 K was equilibrated with air (20% oxygen and 80% nitrogen ) at 1 atm. The dissolved gas was separated from a sample of this water and the dried. Determine the composition of this gas.
1 kg of water under a nitrogen pressure of 1 atmosphere dissolves 0.02 gm of nitrogen at 293 K. Calculate Henry' s law constant :
The Henry's law constant for the solubility of N_(2) gas in water at 298K is 1.0 xx 10^(5) atm. The mole fraction of N_(2) in air is 0.8 . The number of moles of N_(2) from air dissolved in 10 moles of water at 298K and 5 atm pressure is