The organic compound that gives following qualitative analysis is `:`
Test Inference
(a) Dil. HCl Insoluble
(b) NaOH solution soluble
( c ) `Br_(2) //` water Decolourization
The organic compound that gives following qualitative analysis is `:`
Test Inference
(a) Dil. HCl Insoluble
(b) NaOH solution soluble
( c ) `Br_(2) //` water Decolourization
Test Inference
(a) Dil. HCl Insoluble
(b) NaOH solution soluble
( c ) `Br_(2) //` water Decolourization
A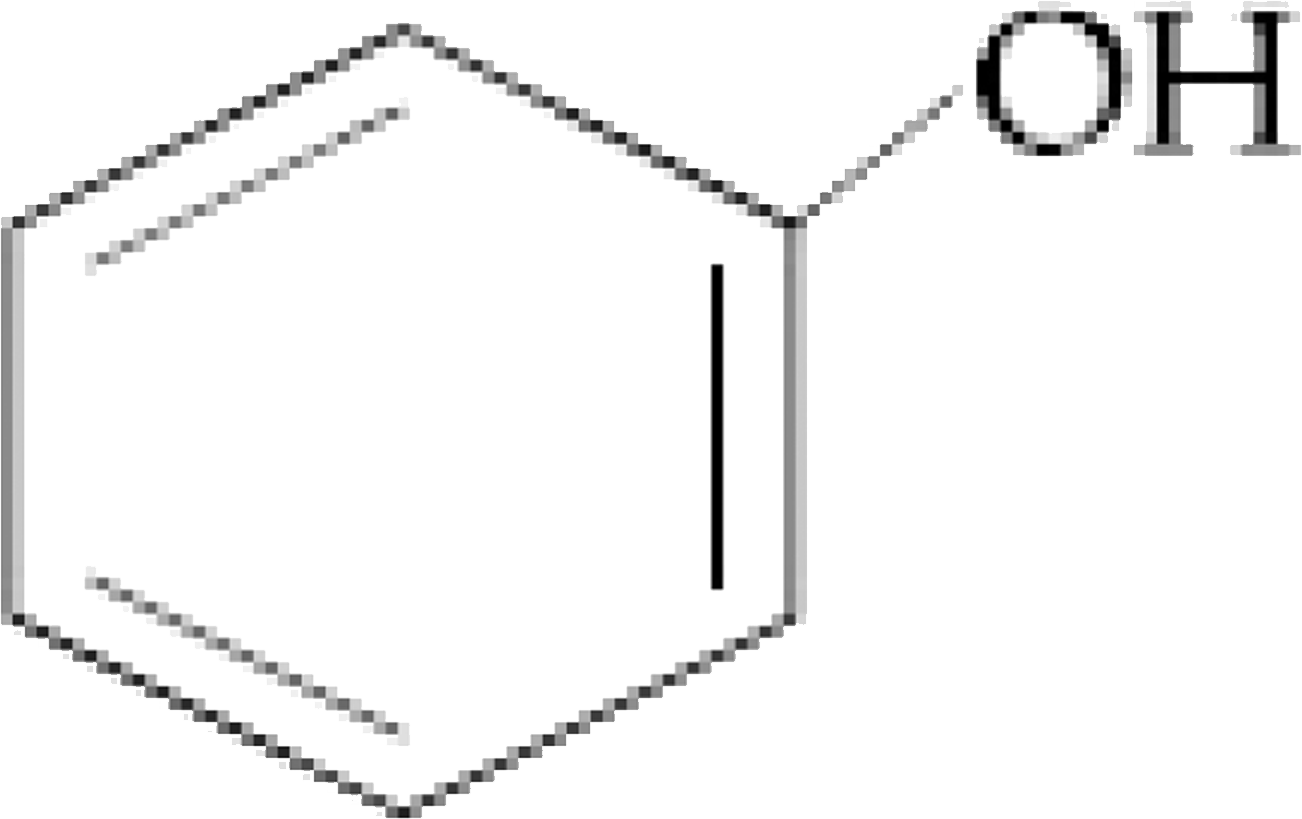

B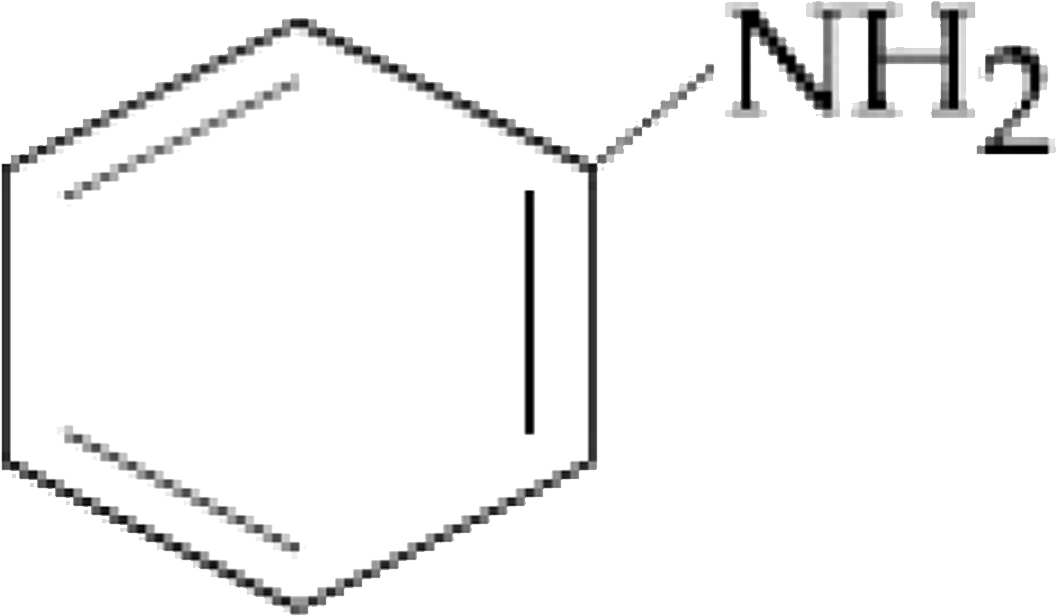

C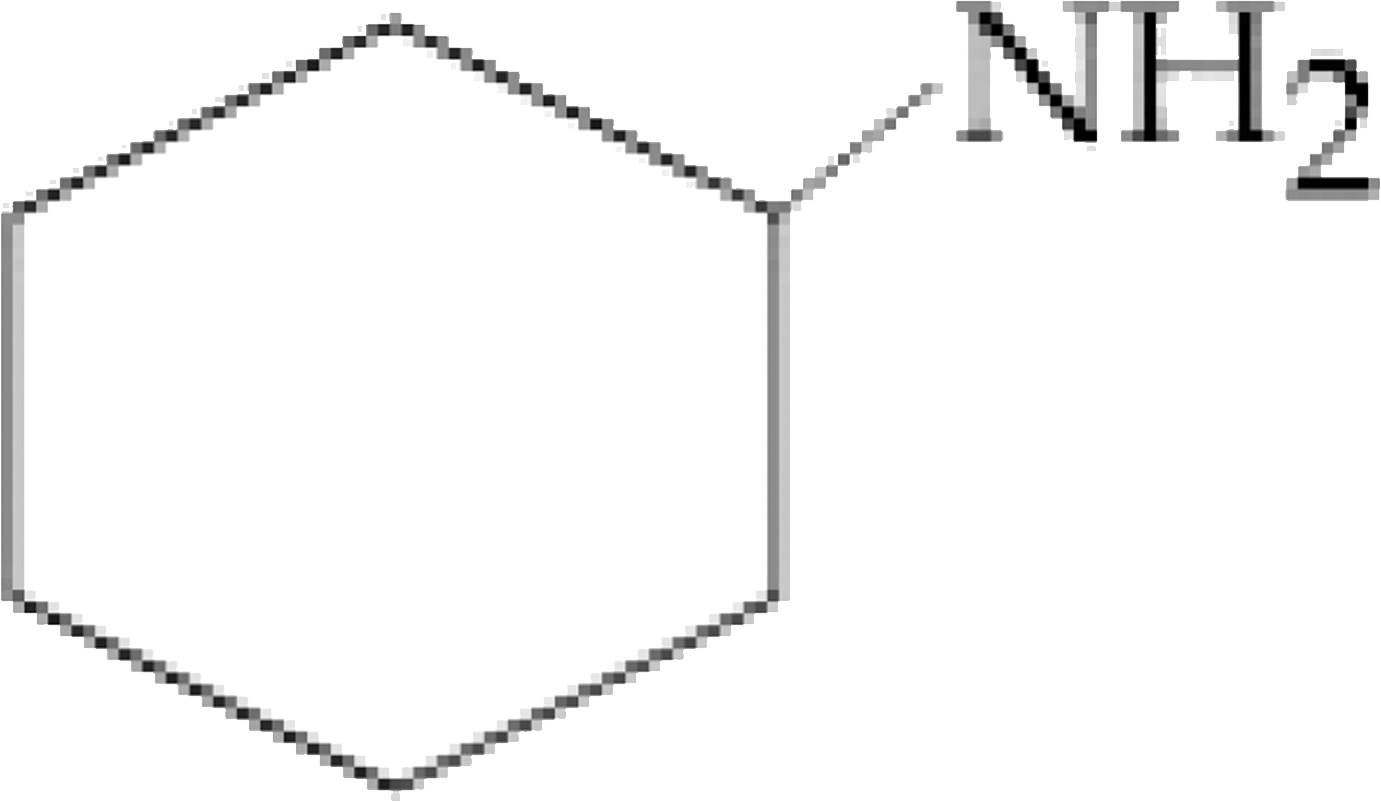

D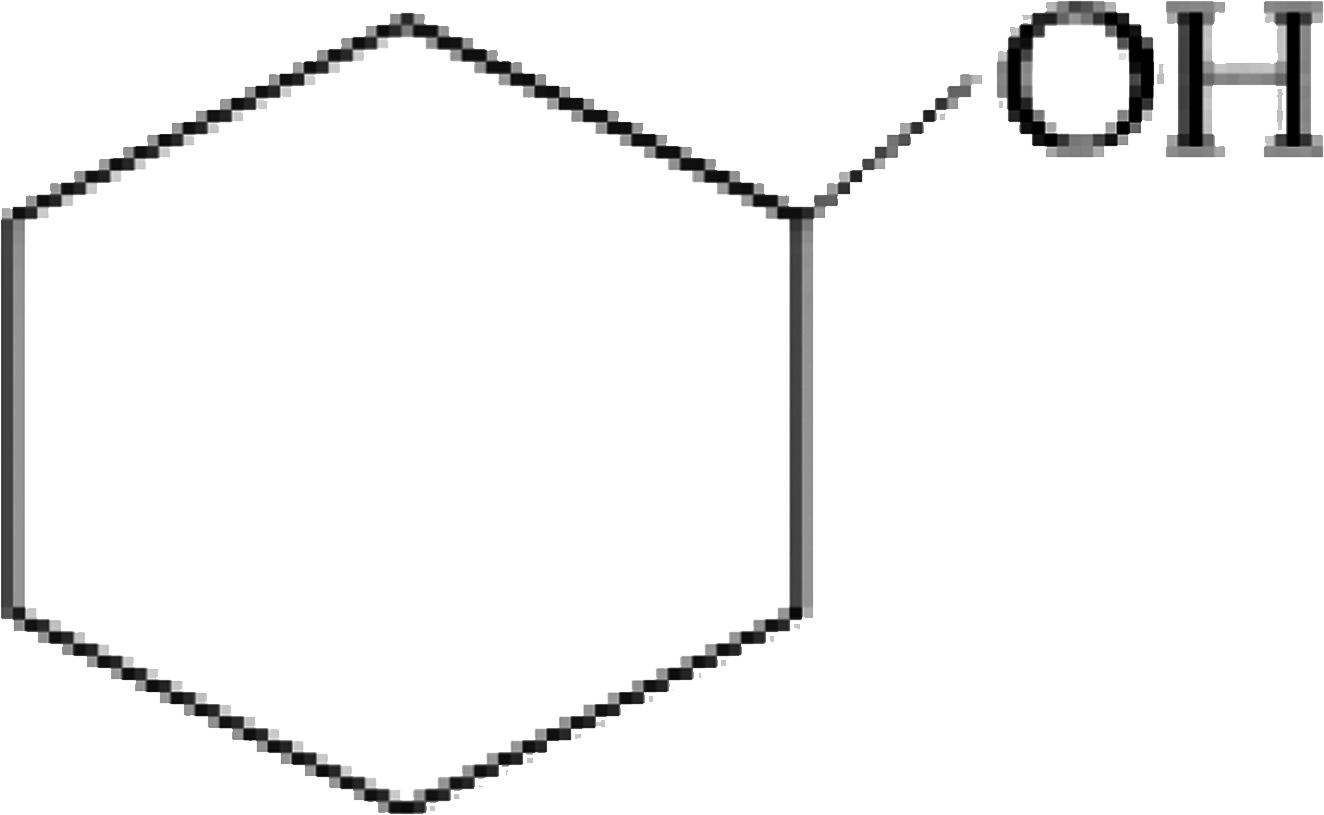

Text Solution
Verified by Experts
The correct Answer is:
A
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
How many of the following compounds decolorise Br_(2) water solution ?
An organic compound containing C, H and O gives following observations: (i) It exists in two isomeric forms (A) and (B). (ii) 0.108 g of one of the isomers on combustion gave 0.308 g of CO_(2) and 0.072 g of H_(2)O (iii) (A) is insoluble in NaOH and NaHCO_(3) while (B) is soluble in NaOH. (iv) (A) reacts with Hl to give compound (C ) and (D). (C ) can be separated from (D) by ethanolic AgNO_(3) solution and (D) is soluble in NaOH. (v) (B) readily reacts with bromine water to give compound (E) of molecular formula C_(7)H_(5)OBr_(3) Compound (B) in the above passage is
An organic compound containing C, H and O gives following observations: (i) It exists in two isomeric forms (A) and (B). (ii) 0.108 g of one of the isomers on combustion gave 0.308 g of CO_(2) and 0.072 g of H_(2)O (iii) (A) is insoluble in NaOH and NaHCO_(3) while (B) is soluble in NaOH. (iv) (A) reacts with Hl to give compound (C ) and (D). (C ) can be separated from (D) by ethanolic AgNO_(3) solution and (D) is soluble in NaOH. (v) (B) readily reacts with bromine water to give compound (E) of molecular formula C_(7)H_(5)OBr_(3) Compound (A) is
An organic compound containing C, H and O gives following observations: (i) It exists in two isomeric forms (A) and (B). (ii) 0.108 g of one of the isomers on combustion gave 0.308 g of CO_(2) and 0.072 g of H_(2)O (iii) (A) is insoluble in NaOH and NaHCO_(3) while (B) is soluble in NaOH. (iv) (A) reacts with Hl to give compound (C ) and (D). (C ) can be separated from (D) by ethanolic AgNO_(3) solution and (D) is soluble in NaOH. (v) (B) readily reacts with bromine water to give compound (E) of molecular formula C_(7)H_(5)OBr_(3) The empirical formula of (A) and (B) is
An organic compound containing C, H and O gives following observations: (i) It exists in two isomeric forms (A) and (B). (ii) 0.108 g of one of the isomers on combustion gave 0.308 g of CO_(2) and 0.072 g of H_(2)O (iii) (A) is insoluble in NaOH and NaHCO_(3) while (B) is soluble in NaOH. (iv) (A) reacts with Hl to give compound (C ) and (D). (C ) can be separated from (D) by ethanolic AgNO_(3) solution and (D) is soluble in NaOH. (v) (B) readily reacts with bromine water to give compound (E) of molecular formula C_(7)H_(5)OBr_(3) Compound (D)underset((iii)HCl)underset(4-7" atm,"125K)overset((i)NaOH)overset((ii)CO_(2))rarr(X)overset(CH_(3)COCl)rarr(Y) The product (Y) is
An organic compound containing C, H and O gives following observations: (i) It exists in two isomeric forms (A) and (B). (ii) 0.108 g of one of the isomers on combustion gave 0.308 g of CO_(2) and 0.072 g of H_(2)O (iii) (A) is insoluble in NaOH and NaHCO_(3) while (B) is soluble in NaOH. (iv) (A) reacts with Hl to give compound (C ) and (D). (C ) can be separated from (D) by ethanolic AgNO_(3) solution and (D) is soluble in NaOH. (v) (B) readily reacts with bromine water to give compound (E) of molecular formula C_(7)H_(5)OBr_(3) Compound (C)overset("Moist"Ag_(2)O)rarr(P)underset(CH_(2)Cl_(2))overset(PC C)rarr(Q) The product (Q) is
Name the organic compound prepared by the following reactions: C_2H_5Br + KOH (alcoholic solution ) to
An element of group 14 form a red coloured mixed oxide (A) which on treatment with conc HNO_3 gives compound (B) , (B) reacts with HCl to produce a choloride (C) , which is insoluble in cold water but soluble in hot water . (A) on reaction with conc HCl produces (C) . Identify (A), (B) and (C) .
An organic compound X (C_(4)H_(8)O_(2)) gives positive test with NaOH and Phenopthalein. Structure of X will be:
Give reasons for the following : MgCl_(2) is soluble in water but insoluble in acetone, while methane is insoluble in water, but soluble in acetone.