A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
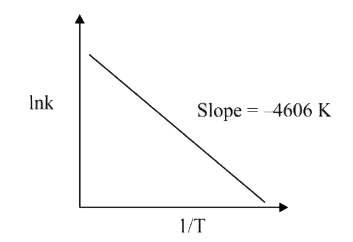
- For a reaction, consider the plot of lnk versus 1/T given in the figur...
Text Solution
|
- For a reaction, consider the plot of In K versus 1//T given in the fig...
Text Solution
|
- The energy of activation of a reaction is 140 kJ "mol"^(-1) . If its r...
Text Solution
|
- A slope of a graph In [A] versus 't' for a first order is -5 xx 10^(-4...
Text Solution
|
- Rate constant of a reaction is k = 3.14 xx 10^(-4) mol L^(-1)s^(-1) . ...
Text Solution
|
- The rate constants of a reaction at 500 K and 700 K are 0.02 s^(–1) an...
Text Solution
|
- At 407 K the rate constant of a chemical reaction is 9.5xx10^(-5)s^(-1...
Text Solution
|
- The rate constants for a reaction at 400 K and 500 K are 2 . 60 xx 10^...
Text Solution
|
- The rate constant of a first order reaction at 500 K is 2.35 xx 10^(-5...
Text Solution
|