Compound (A) `C_(8)H_(9)Br`. Gives a white precipitate when warmed with alcoholic `AgNO_(3)`. Oxidation of (A) gives an acid (B). `C_(8)H_(6)O_(4)`. (B) easily forms anhydride on heating. Identify the compound (A)
Compound (A) `C_(8)H_(9)Br`. Gives a white precipitate when warmed with alcoholic `AgNO_(3)`. Oxidation of (A) gives an acid (B). `C_(8)H_(6)O_(4)`. (B) easily forms anhydride on heating. Identify the compound (A)
A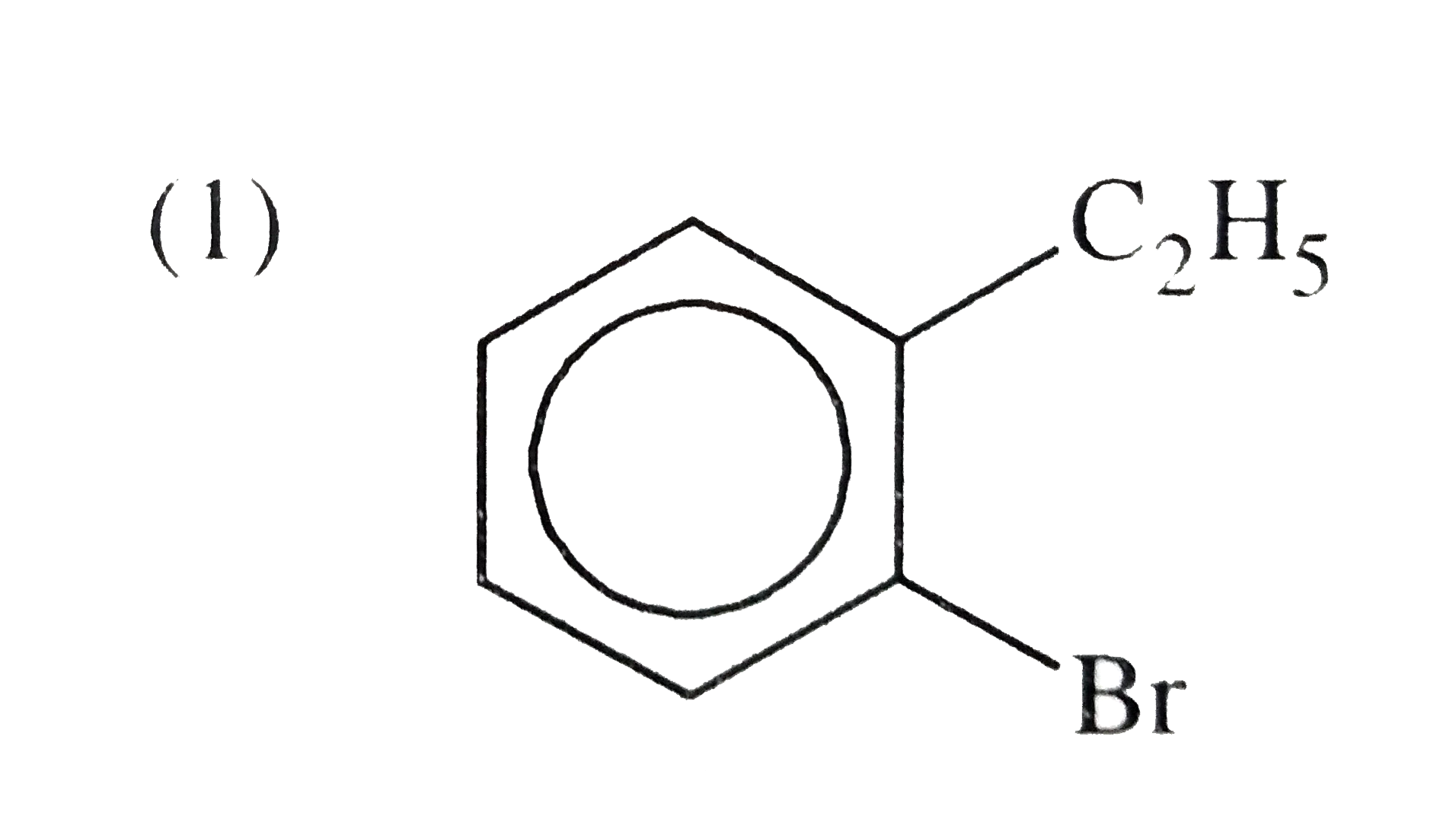

B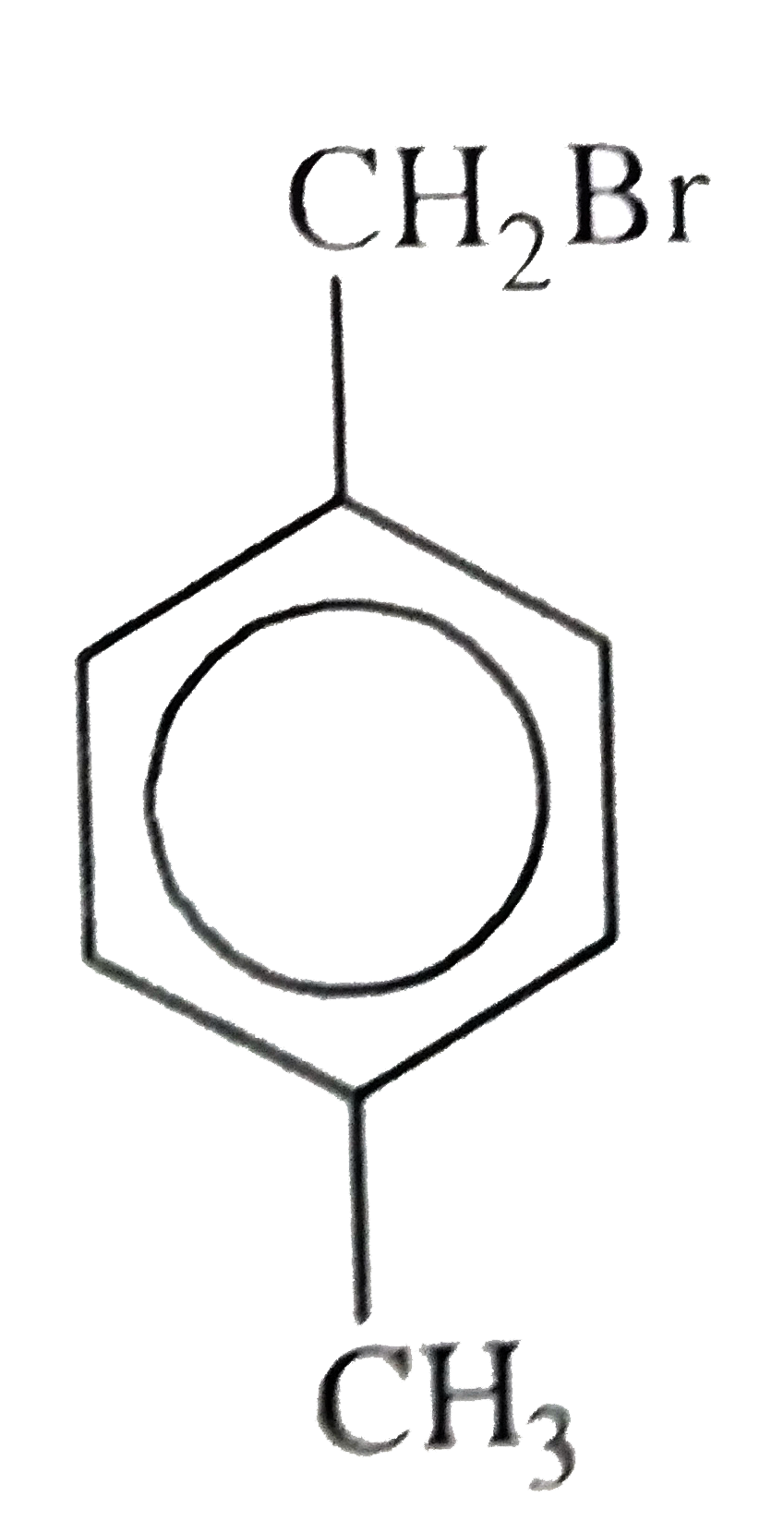

C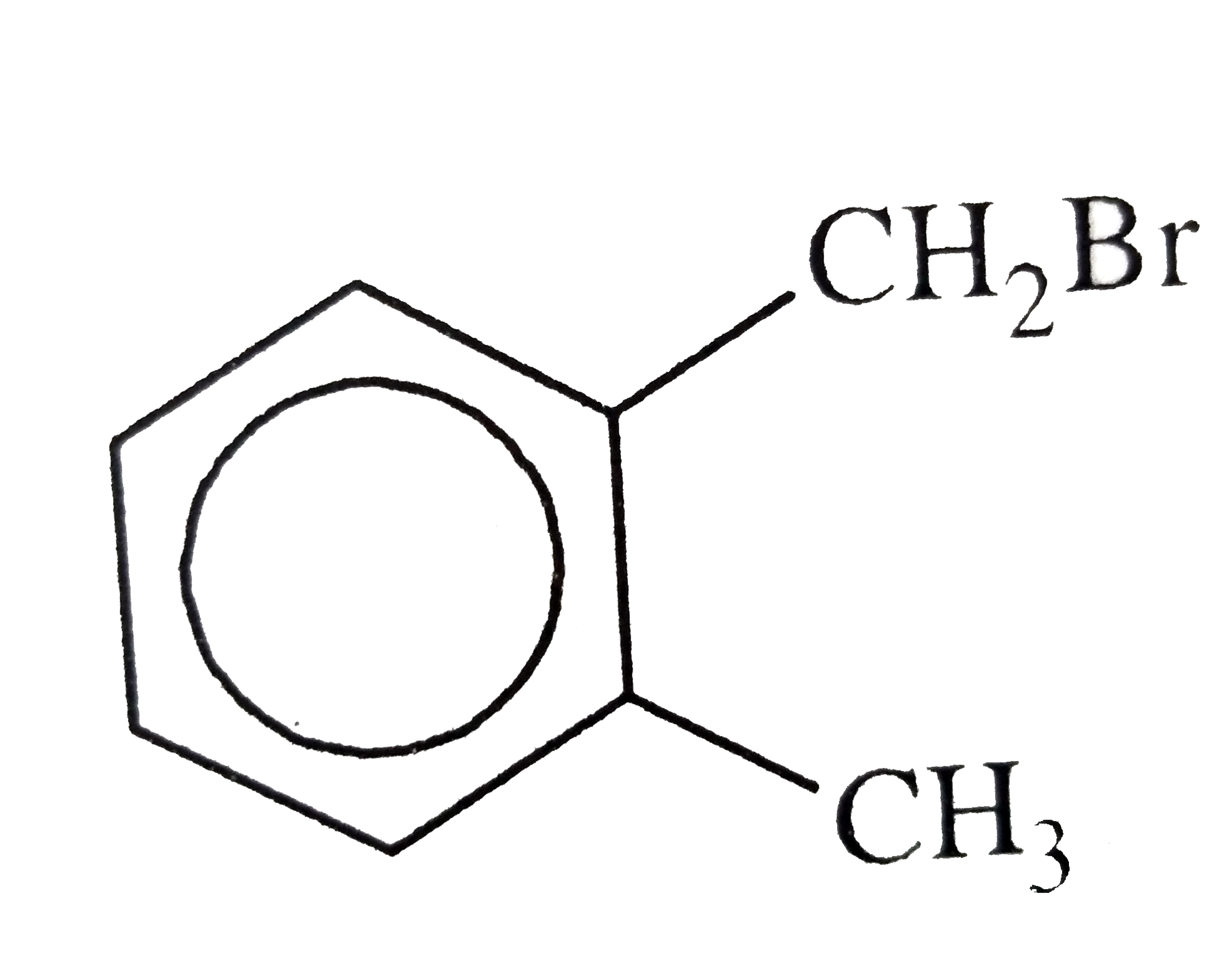

D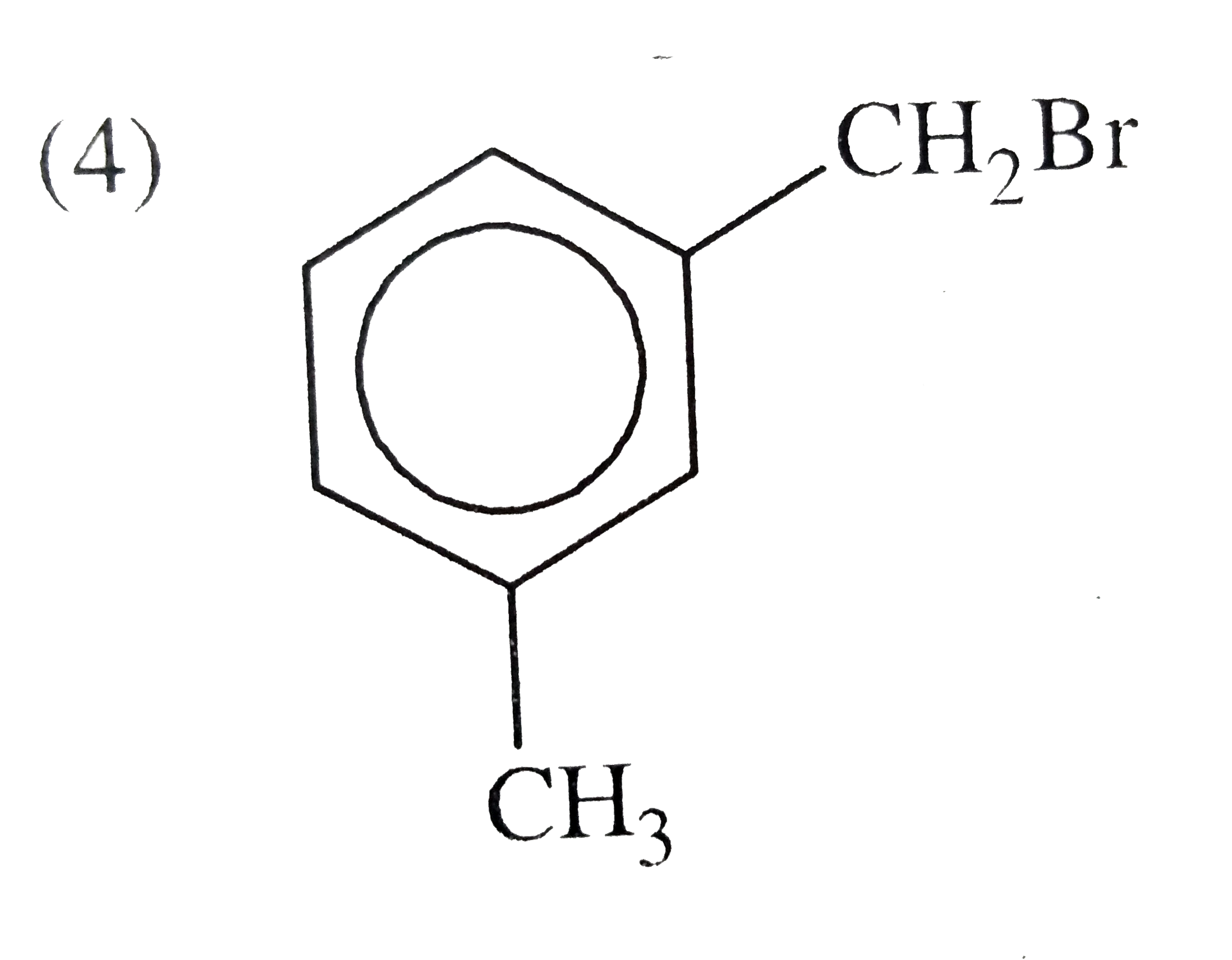

Text Solution
AI Generated Solution
The correct Answer is:
To identify compound (A) with the molecular formula \( C_8H_9Br \), we need to analyze the information given in the question step by step.
### Step 1: Analyze the Reaction with Alcoholic AgNO3
The compound (A) gives a white precipitate when warmed with alcoholic \( AgNO_3 \). This indicates that (A) contains a bromine atom that can be replaced by the alkoxy group from the alcohol in the reaction. The precipitate formed is \( AgBr \), which is typically light yellow.
### Step 2: Determine the Structure of Compound (A)
Given the molecular formula \( C_8H_9Br \), we can deduce that (A) likely contains a bromine atom attached to a carbon chain or a ring structure. The presence of 9 hydrogens suggests that there may be a double bond or a ring structure, as each bromine substitution reduces the number of hydrogens.
### Step 3: Oxidation of Compound (A)
The oxidation of (A) produces an acid (B) with the formula \( C_8H_6O_4 \). This indicates that upon oxidation, two hydrogen atoms are replaced with carboxylic acid groups (\(-COOH\)).
### Step 4: Identify the Structure of Acid (B)
The molecular formula \( C_8H_6O_4 \) suggests that (B) has two carboxylic acid groups. Since it can easily form an anhydride upon heating, it is likely that (B) is phthalic acid, which has the structure:
\[
\text{C}_6\text{H}_4(\text{COOH})_2
\]
### Step 5: Identify Compound (A) Based on the Structure of (B)
To form phthalic acid from (A), (A) must be a bromo derivative of a compound that can oxidize to form two carboxylic acid groups. The most likely candidate is 1-bromo-2-methylbenzene (o-bromotoluene), which has the structure:
\[
\text{C}_6\text{H}_4(\text{Br})(\text{CH}_3)
\]
### Step 6: Confirm the Structure of (A)
1. **Reactivity with \( AgNO_3 \)**: The bromo group in 1-bromo-2-methylbenzene will react with alcoholic \( AgNO_3 \) to form \( AgBr \).
2. **Oxidation**: Upon oxidation, the methyl group can be converted to a carboxylic acid, yielding phthalic acid.
3. **Formation of Anhydride**: Phthalic acid can easily lose water to form phthalic anhydride upon heating.
### Conclusion
Thus, the compound (A) is identified as **1-bromo-2-methylbenzene**.
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
C Cl_4 gives a white precipitate when treated with AgNO_3 .
Compound (A) ( C_(6)H_(8))overset([o])(rarr) Malonic acid only. Identify compound (A) .
An organic compound (A) (C_(8)H_(14)O) forms an oxime and gives a positive haloform reaction. On ozonolysis, it gives acetone and a compound (B) (C_(5)H_(8)O_(2)) . (B) forms a dioxime and on subjecting to oxidation reaction gives an acid (C ) (C_(4)H_(6)O_(4)) . On treatment with excess of ammonia and strong heating, (C ) gives a neutral compound (D) (C_(4)H_(5)O_(2)N) . (D) on distillation with zinc dust forms pyrrloe. Suggest the possible structures of (A), (B), (C ), and (D). Explain the chemical reactions involved.
A compound (C_(5)H_(8)) reacts with ammoniacal AgNO_(3) to give a white precipitate and reacts with excess of KMnO_(4) solution to give (CH_(3))_(2)CH-COOH . The compound is
Compound 'A' with the formula C_(3)H_(8)O on vigorous oxidation produces an acid C_(3)H_(6)O_(2) . 'A' is
Compound A(C_(9)H_(10)O) shows positive iodoform test. Oxidation of A with KMnO_(4)//KOH gives acid B(C_(8)H_(6)O_(4)) . Anhydride of B is used for the preparation of phenolphthalein. Compound A is:
An organic compound (A) (C_(8)H_(8)O_(3)) was insoluble in water, dilute HCI, and NaHCO_(3) . It was soluble in NaOH. A solution of (A) in dilute NaOH was boiled and steam distilled and distillate was reacted with NaOH to give a yellow precipitate. The alkaline residue is acidifield to give a solid (B) (C_(7)H_(6)O_(3)) . (B) dissolves in aqueous NaHCO_(3) with the evolution of gas. Identify (A) and (B).
Compound (C_(9)H_(10)O) shows positive iodoform test. Oxidation of A with KMnO_(4)//KOH gives acid B(C_(8)H_(6)O_(4)) anhydride of B is used for the preparation of phenolphthalein compound is
The compound C_8H_9Cl(A) on treatments with KCN followed by hydrolysis gives C_9H_10O_2(B) .Ammonium salt of B on dry distillation yields C.Which reacts with alkaline solution of bromine to gives C_8H_11N .(D),Another compound E (C_6H_10O) is obtained by the action of nitrous acid on D. or by the action of aquouspotash on A,E on oxidation gives F(C_8H_10O) Which gives the inner anhydride G on heating. The compound D is reaction with CHCl_3+NaOH gives a compound H. The compound A on reaction with AgCN gives :
The compound C_8H_9Cl(A) on treatments with KCN followed by hydrolysis gives C_9H_10O_2(B) .Ammonium salt of B on dry distillation yields C.Which reacts with alkaline solution of bromine to gives C_8H_11N .(D),Another compound E (C_6H_10O) is obtained by the action of nitrous acid on D. or by the action of aquouspotash on A,E on oxidation gives F(C_8H_10O) Which gives the inner anhydride G on heating. The compound D is reaction with CHCl_3+NaOH gives a compound H.The structure of H.