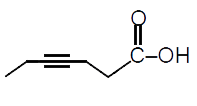
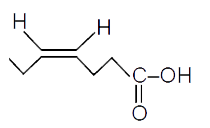
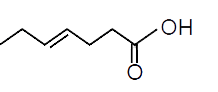
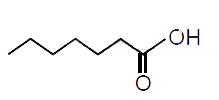A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-CHEMISTRY
- "Hex-3-ynal" overset((1)NaBH(4))to overset((2)PBr(3))to overset((3)"Mg...
Text Solution
|
- E(Cu^(2+)//Cu)^(@)=0.34V E(Cu^(+)//Cu)^(@)=0.522V E(Cu^(2+)//Cu^(+...
Text Solution
|
- The number of orbitals associated with quantum numbers n =5, ...
Text Solution
|
- Arrange the following in order of their pK(b) value (a) (b) CH(3)-...
Text Solution
|
- The electron gain enthalpy ( in kJ//mol ) of fluorine, chlorine,...
Text Solution
|
- A solution of m - chloroaniline, m- chlorophenol and m - chlorobenz...
Text Solution
|
- Atomic radius of Ag is similar to
Text Solution
|
- Which theory can explain bonding Ni ( CO ) 4
Text Solution
|
- Amongst the following statements, that which was not proposed D...
Text Solution
|
- Consider the following reactions : (a) ( CH 3) 3 CCH ( O...
Text Solution
|
- Correct IUPAC name of [ Pt ( N H 3 ) 2 ) Cl ( CH 3 NH 2...
Text Solution
|
- In zeolites & synthetic resin method which will be more efficient...
Text Solution
|
- Consider the following reaction : The product 'X' is used...
Text Solution
|
- The dipole moments of CCl 4, CHCl 3 and CH 4 are i...
Text Solution
|
- 1-"Methylethylene oxide" underset(HBr)overset("excess")to X, Product '...
Text Solution
|
- "Hex-3-ynal" overset((1)NaBH(4))to overset((2)PBr(3))to overset((3)"Mg...
Text Solution
|
- Vapour pressure of pure CS 2 and CH 3 COCH 3 are 512 ...
Text Solution
|
- Oxidation number of potassium in K 2 O, K2 O 2 and K O ...
Text Solution
|
- The relative strength of interionic / intermolecular forces in d...
Text Solution
|
- Match the following :
Text Solution
|
- Purest form of commercial iron is :
Text Solution
|