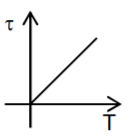
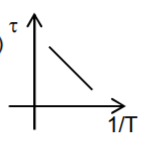
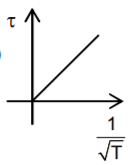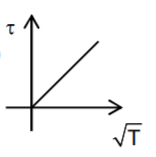A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-All Questions
- Choose the correct graph between pressure and volume of ideal gas.
Text Solution
|
- Find the co-ordinates of centre of mass of the lamina shown in figure
Text Solution
|
- Which graph correctly represents variation between relaxation time (t)...
Text Solution
|
- If two capacitors C1 & C2 are connected in parallel then equivalent ca...
Text Solution
|
- A rod of mass 4m and length L is hinged at the mid point. A ball of ma...
Text Solution
|
- When photon oFIGURE energy 4.0eV strikes the surFIGUREace oFIGURE a me...
Text Solution
|
- The length oFIGURE a potentiometer wire is 1200cm and it carries a cur...
Text Solution
|
- A telescope has magnification 5 and length of tube 60cm then the focal...
Text Solution
|
- Two spherical bodies of mass m1 & m2 are having radius 1 m & 2 m respe...
Text Solution
|
- When proton of KE = 1.0 MeV moving in South to North direction enters ...
Text Solution
|
- If electric field around a surface is given by |vec(E)|=(Q(in))/(epsil...
Text Solution
|
- Stopping potential depends on planks constant (h), current (I), univer...
Text Solution
|
- A cylinder of height 1m is floating in water at 0^@C with 20cm height ...
Text Solution
|
- Number of the alpha- particle deflected in Rutherford's alpha -scatter...
Text Solution
|
- If relative permittivity and relative permeability of a medium are 3 a...
Text Solution
|
- The given loop is kept in a uniform magnetic field perpendicular to pl...
Text Solution
|
- Choose the correct Boolean expression for the given circuit diagram:
Text Solution
|
- A Solid sphere of density rho=rho0(1-r^2/R^2), 0 ltrleR just floats in...
Text Solution
|
- Two masses each with mass 0.10kg are moving with velocities 3m/s along...
Text Solution
|
- A plano convex lens of radius of curvature 30 cm and refractive index ...
Text Solution
|