A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
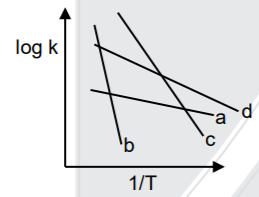
- Compare Ea (activation energy) for a, b, c and d.
Text Solution
|
- Compare Ea (activation energy) for a, b, c and d.
Text Solution
|
- साधारण रासायनिक अभिक्रिया ArarrB के लिए सक्रियण ऊर्जा, अग्र अभिक्रिया...
Text Solution
|
- For the exothermic reaction, A + B to C +D. Delta H is the heat of rea...
Text Solution
|
- नाभिकीय अभिक्रिया A + B =3D C + D में मुक्त ऊर्जा का मान ज्ञात करें मा...
Text Solution
|
- The reaction A+B rarr C+D+40KJ has activation energy of "18KJ" .Then ...
Text Solution
|
- The activation energy for a simple chemical reaction A rarr B is E(a) ...
Text Solution
|
- A : For exothermic reaction, DeltaH=Ea (forward) -Ea (backward) R : Th...
Text Solution
|
- The activation energy for a simple chemical reaction A rarr B is E(a) ...
Text Solution
|