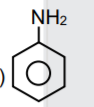
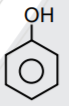
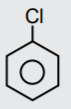
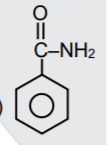
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following can give highest yield in Friedel craft reactio...
Text Solution
|
- Which of the following compound will not give Friedel-Crafts reaction?
Text Solution
|
- Which of the following can be used as halide component for Friedel-Cra...
Text Solution
|
- Which of the following does not gives Friedel-Crafts reaction ?
Text Solution
|
- Which of the following does not gives Friedel-Crafts reaction ?
Text Solution
|
- Which of the following compounds do not give Friedel Crafts reaction?
Text Solution
|
- Which of the following can give highest yield in Friedel craft reactio...
Text Solution
|
- निम्न यौगिकों में से कौन सी फ्रीडेल-क्राफ्ट्स अभिक्रिया आसानी से नहीं ...
Text Solution
|
- Which of the following can be used as the halide component for Friedel...
Text Solution
|