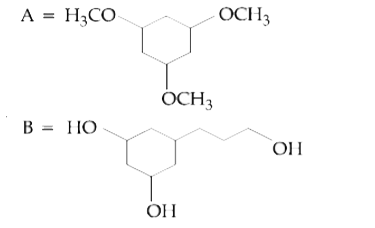
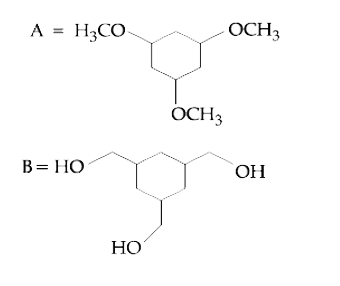
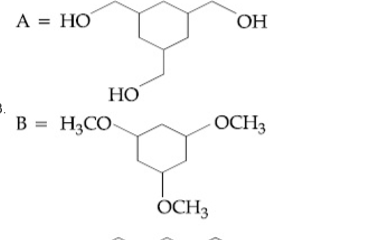
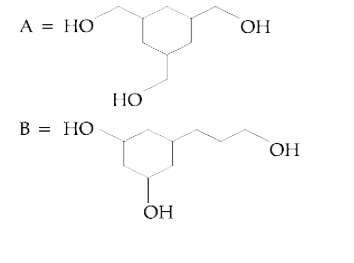
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-CHEMISTRY
- Assertion: It has been found that for hydrogenation reaction the catal...
Text Solution
|
- The major product [B] in the following seqyence of reaction is :
Text Solution
|
- There are two compounds A and B of molecular formula C(9)H(18)O(3). A ...
Text Solution
|
- A metal (A) on heating in nitrogen gas given compound B.B on terat...
Text Solution
|
- Tow monomers in maltose are :
Text Solution
|
- Which reaction does not occurs in the blast furnace in the metallurgy ...
Text Solution
|
- Among (a) -(d) the complexes that can display geometical isomerism ar...
Text Solution
|
- The increasing order of the atomic radii of the following element...
Text Solution
|
- How many atoms lie in the same plane in the major product (C) ? (Wher...
Text Solution
|
- Complex [ML(5)] can exhibit trigonal bipyramidal and square pyramidal ...
Text Solution
|
- At constant volume 4 mol of an ideal gas when heated form 300k to 50...
Text Solution
|
- NaClO(3) is used even is spacecrafts to produce O(2) . The daily con...
Text Solution
|
- Given: E(Sn^(2+)//Sn) ^0 = -0.14V , E(Pb^(2+)//Pb) ^0 = -0.13V. Determ...
Text Solution
|
- The electronic configuration of bivalent Europium and trivalent cerium...
Text Solution
|
- If the magnetic moment of a dioxygen species is 1.73 B.M, it may be:
Text Solution
|
- The major product (Y) in the following reactions is: CH(3)-overset(C...
Text Solution
|
- The major product Z obtained in the following reaction scheme is:
Text Solution
|
- The increasing order of basicity for the following intermediates is (f...
Text Solution
|
- Complex Cr(H2 O)6 Cln shows geometrical isomerism and also reacts with...
Text Solution
|
- According to the following diagram, A reduces BO(2) when the temperatu...
Text Solution
|