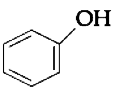
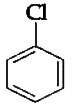
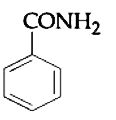
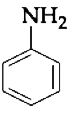
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-CHEMISTRY
- Which of the following can not act as both oxidising and reducing agen...
Text Solution
|
- Which of the following is correct order for heat of combustion?
Text Solution
|
- Which of the following can give highest yield in Friedel craft reactio...
Text Solution
|
- The de-Broglie wavelength of an electron in 4th orbit is (where, r=rad...
Text Solution
|
- For Br2(l) Enthalpy of atomisation = x kJ/mol, Bond dissociation entha...
Text Solution
|
- Determine degree of hardness in term of ppm of CaCO3 of 10^(-3) molar ...
Text Solution
|
- Given a solution of HNO3 of density 1.4 g/mL and 63% w/w. Determine mo...
Text Solution
|
- Find percentage nitrogen by mass in Histamine?
Text Solution
|
- Determine the amount of NaCl to be dissolved in 600g H(2)O to decrease...
Text Solution
|
- On passing a particular amount of electricity in AgNO3 solution, 108 g...
Text Solution
|
- In the following reaction A is :
Text Solution
|
- Which of the following reaction will not form racemic mixture as produ...
Text Solution
|
- Amongst the following , the form of water with the lowest ionic conduc...
Text Solution
|
- Biochemical oxygen demand (BOD) is defined as ............ in ppm of O...
Text Solution
|
- Among the statements (a)-(d) , the correct ones are : (a) Lithium ha...
Text Solution
|
- The decreasing order of basicity of the following amines is :
Text Solution
|
- Given following complexes (I) Na4[Fe(CN)6] (II) [Cr(H2O)6] Cl2 (III)...
Text Solution
|
- Given K(sp) for Cr(OH)3 is 6 xx 10^(-31) then determine [OH^- ]. (Negl...
Text Solution
|
- Number of sp^2 hybrid orbitals in Benzene is
Text Solution
|
- The reaction of H(3)N(3)B(3)Cl(3)(A) with LiBH(4) in tetrahydrofuran g...
Text Solution
|