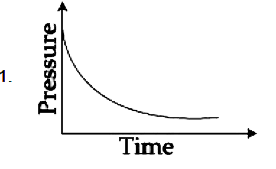
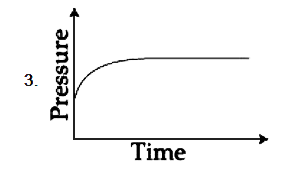
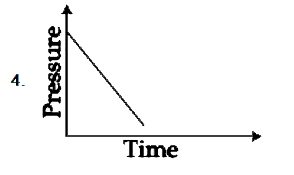
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-CHEMISTRY
- Among the statements (a)-(d) , the correct ones are : (a) Lithium ha...
Text Solution
|
- The decreasing order of basicity of the following amines is :
Text Solution
|
- Given following complexes (I) Na4[Fe(CN)6] (II) [Cr(H2O)6] Cl2 (III)...
Text Solution
|
- Given K(sp) for Cr(OH)3 is 6 xx 10^(-31) then determine [OH^- ]. (Negl...
Text Solution
|
- Number of sp^2 hybrid orbitals in Benzene is
Text Solution
|
- The reaction of H(3)N(3)B(3)Cl(3)(A) with LiBH(4) in tetrahydrofuran g...
Text Solution
|
- In a box a mixture containing H2, O2 and CO along with charcoal is pre...
Text Solution
|
- Monomer(s) of which of the given polymer is chiral?
Text Solution
|
- Given complex [Co(NH3)4Cl2]. In it if Cl – Co – Cl bond angle is 90^@ ...
Text Solution
|
- Reactant A represented by square is in equilibrium with product B repr...
Text Solution
|
- Consider the following reactions The compound [P] is :
Text Solution
|
- The true statement amongst the following is :
Text Solution
|
- In which compound C–Cl bond length is shortest
Text Solution
|
- When same amount of zinc is treated separately with excess of H(2)SO(4...
Text Solution
|
- Given an element having following ionisation enthalpies IE1 = 496 (kJ)...
Text Solution
|
- Total number of Cr–O bonds in Chromate ion and dichromate ion is.
Text Solution
|
- Lacto bacillus has generation time 60 min. at 300 K and 40 min. at 400...
Text Solution
|
- 0.1 mole of an ideal gas has volume 1 dm^3 in a locked box with fricti...
Text Solution
|
- Consider the following reactions A underset((ii)H(3)O^(+))overset((...
Text Solution
|
- One litre sea water (d = 1.03g/cm^3 ) contains 10.3 mg O2 gas. Determi...
Text Solution
|