A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
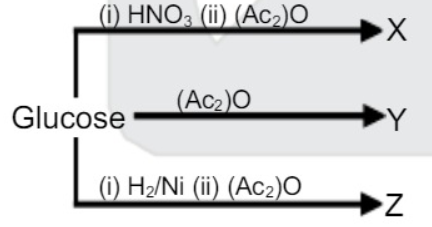
- calculate require moles of (Ac)2O IN X,Y,Z product formation
Text Solution
|
- Identify the product Z in the following reacton C6H5NH2overset((AC)2O)...
Text Solution
|
- Calculate the minimum number of moles of R - Mg - X (Grignard reagent)...
Text Solution
|
- x,y,z araea mole used sum of [X+Y+Z=]
Text Solution
|
- 10 moles of X, 12 mole of Y and 20 moles of Z are mixed to produce a f...
Text Solution
|
- Three acyclic alkenes (x,y,z) on catalytic hyrogenation give same alka...
Text Solution
|
- If x+z = 2y " and " b^2 = ac, then prove that a^(y-z)*b^(z-x)*c^(x-y) ...
Text Solution
|
- 5 mole of X are mixed with 3 moles of Y. At equilibrium for the reacti...
Text Solution
|
- calculate require moles of (Ac)2O IN X,Y,Z product formation
Text Solution
|