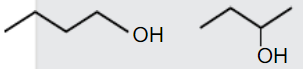
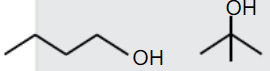
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two acylic compound A & B having same molecular formula C3H6O. A & B r...
Text Solution
|
- A,B,C and D have position vectors a,b,c and d, respectively, such that...
Text Solution
|
- Identify the A,B,C and D are, respectively .
Text Solution
|
- Two acylic compound A & B having same molecular formula C3H6O . A & B ...
Text Solution
|
- Two isomers (A) and (B) have the same molecular formula C2H4Cl2. Comp...
Text Solution
|
- An organic compound A of molecular formula CH2O reacts with methyl ma...
Text Solution
|
- An organic compound (A) of molecular formula C6H6reacts with propylene...
Text Solution
|
- A Compound (A) with molecular formula C,H,N on acid hydrolysis gives(B...
Text Solution
|
- An organic compound (A) is a calcium salt of acetic acid. (A) on dry d...
Text Solution
|