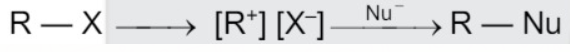A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- which statement is/are correct for this reaction? (I) polarity of so...
Text Solution
|
- Nucleophilic aliphatic substitution reaction is mainly of two types: S...
Text Solution
|
- In which of the above reactions does the rate fo SN^(2) reactions decr...
Text Solution
|
- Assertion (A) : SN^(1) reaction is carried out in the presence of a pl...
Text Solution
|
- दो या अधिक सोपानों में सम्पन होने वाली अभिक्रियाएं अभिक्रियाएं कहलाती...
Text Solution
|
- Statement-1 : The overall rate of a reversible reaction may decrease w...
Text Solution
|
- which statement is/are correct for this reaction? (I) polarity of solv...
Text Solution
|
- Consider the ollowing statement about SN^(1) reaction (i) it is a bi...
Text Solution
|
- As ............ is lowered in the presence of catalyst, more molecules...
Text Solution
|