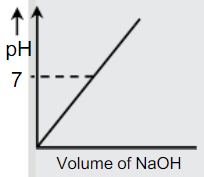
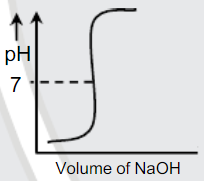
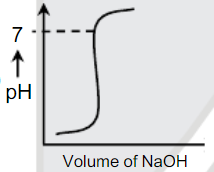
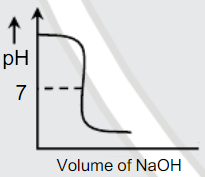
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In 0.1M HCl solution, 0.1M NaOH solution is added gradually then ident...
Text Solution
|
- 0.1M HCl is added to an unknown strength of NaOH solution. Identify th...
Text Solution
|
- Calcualte the pH at the equivalence point when a solution of 0.1M acet...
Text Solution
|
- The pH of a solution obtained by mixing 100mL of 0.1M HCI and 9.9mL of...
Text Solution
|
- A solution of weak acid was titrated with base NaOH. The equivalence p...
Text Solution
|
- 50mL of 0.05M Propane -1,2- diamine solution is titrated with 0.1M HCl...
Text Solution
|
- 25 ml of the given HCl solution requires 30 mL of 0.1M sodium carbonat...
Text Solution
|
- 0.1M HCl तथा 0.01M NaOH में से किस विलयन का pH मान अधिक होगा ?
Text Solution
|
- Calculate the pH at the equivalence point when a solution of 0.1M acet...
Text Solution
|