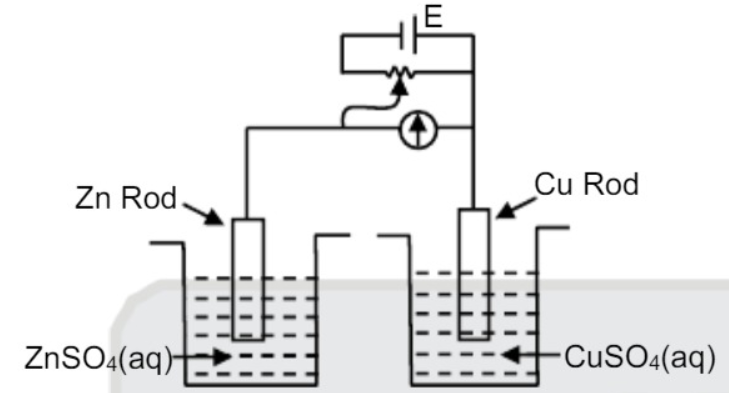
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the given cell arrangement identify incorrect statement given E^...
Text Solution
|
- Construct a cell using given electrodes at 298K and also calculate its...
Text Solution
|
- Given that E^(@)(Zn) = -0.76V and E^(@)(Cu) = 0.34V. Calculate the emf...
Text Solution
|
- Determine the maximum amount of work that can be derived from the cell...
Text Solution
|
- For the given cell arrangement identify incorrect statement given E^@(...
Text Solution
|
- E^(@) of Cu is +0.34V while that of Zn is -0.76V. Explain
Text Solution
|
- For the given cell arrangement identify incorrect statement given E^...
Text Solution
|
- (a) Calculate DeltaG^(@) for the reaction Zn(s) +Cu^(2+) (aq) to Zn^(2...
Text Solution
|
- Determine true statement for Zn-Cu electrochemical cell. [E(Zn^(+2)|Zn...
Text Solution
|