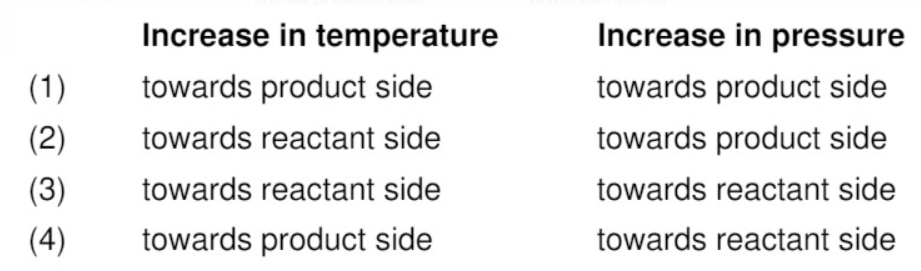
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the following reaction at equilibrium 2NO2(g) harr N2O4(g) Delta...
Text Solution
|
- Which of the following change will shift the reaction in forward direc...
Text Solution
|
- N2O4(g) hArr 2NO2(g) . In this reaction, NO2 is 20% of the total volum...
Text Solution
|
- In the following equilibrium , N2O4(g) hArr 2NO2(g) When 5 moles of ea...
Text Solution
|
- For the following equilibrium reaction , N2O4(g) hArr 2NO2(g) NO2 ...
Text Solution
|
- For the following reaction at equilibrium 2NO2(g) harr N2O4(g) Delta...
Text Solution
|
- 2H2(g) +CO(g) hArr CH3OH(g) , DeltaH=-92.2 kJ. Which of the following ...
Text Solution
|
- In which of the following reactions, increase in temperature as well a...
Text Solution
|
- For the equilibrium reaction 2NO2(g) hArr N2O4(g) + 60.0 kJ the incr...
Text Solution
|