Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SL ARORA-Thermal Properties of Matter-Exercise
- The critical temperature of water is the
Text Solution
|
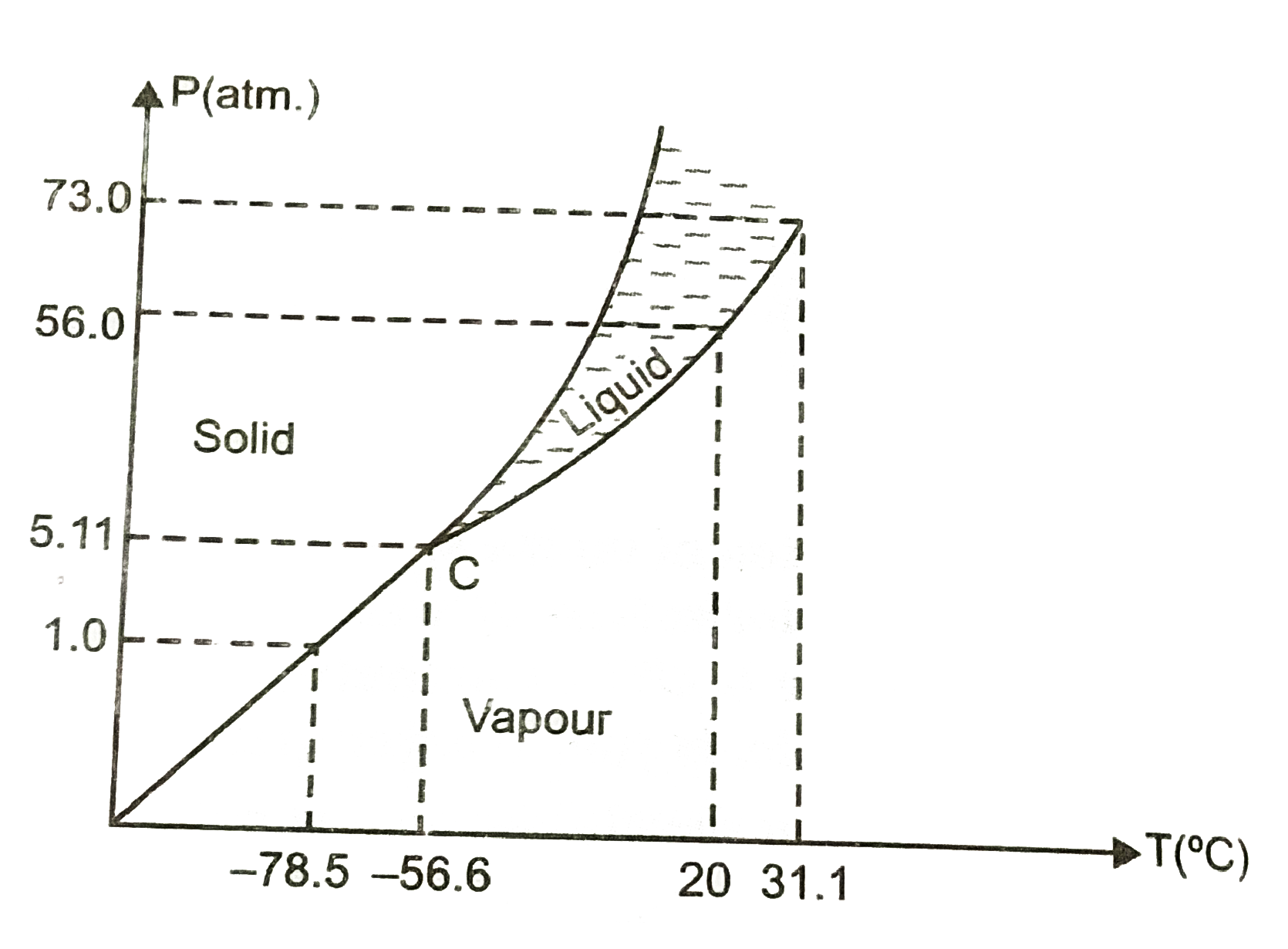
- Answer the following question beased on tbhe P-T phase diagram of carb...
Text Solution
|
- Answer the following question beased on tbhe P-T phase diagram of carb...
Text Solution
|
- Answer the following questions based on the P – T phase diagram of CO(...
Text Solution
|
- Answer the following questions based on the P – T phase diagram of CO(...
Text Solution
|
- Draw the graph showing cooling of hot water with time.
Text Solution
|
- Distinguish between heat and temperature.
Text Solution
|
- What is meant by the statement that heat is an energy in transit ?
Text Solution
|
- Why is mercury used in thermometers ?
Text Solution
|
- State Joule's law of equivalence between work and heat. Hence define m...
Text Solution
|
- Define ideal gas temperature. Does it depend on the nature of the gas ...
Text Solution
|
- What is a liquid thermometer ? Briefly describe its working principle.
Text Solution
|
- Describe the working principle of a platinum resistance thermometer.
Text Solution
|
- How does the coefficient of cubical expansion of a substance vary with...
Text Solution
|
- Prove that the coefficient of cubical expansion of an ideal gas at con...
Text Solution
|
- How does the density of a solid or liquid change with temperature ? Sh...
Text Solution
|
- Mention two practical applications of thermal expansion in daily life.
Text Solution
|
- Define latent heat of fusion of ice and latent heat of vaporisation of...
Text Solution
|
- Define terms heat current and thermal resistance. Write their SI units...
Text Solution
|
- Define thermal resistance. On what factors does it depend ? Deduce its...
Text Solution
|