Observation
(i) most of the `alpha`- particles passed through the gold foil undeflected.
(ii) A small fraction of the `alpha` particles was deflected by small angles
(iii) A very few `alpha-` particles bounced back that is were deflected by nearly `180^(@)`
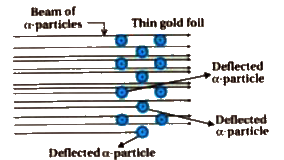
conclusions on the basis of `alpha-` particle scattering experiment.
(i) Most of the space in the atom empty as most of the `alpha-` particles passed through the foil undeflected
(ii) A few positively charged `alpha`- particles wered deflected . The deflection must be due to emomous repulsive force showing that the positive charge of the atom is not spread presumed . the positive charge the has to be concentrated in a very small volume that repelled and deflected the positive charge `alpha` particles
(ii) Calculations by Rutherford showed that the volume occupied by the nucleus is negliglibly small as compared to the total volume of the atom the radius of the atom is about `10^(-10)` while that of nucleus is `10^(15)m` One can appreciate this difference is size by realising that if a cricket ball atom would be about 5 km .
