Photoelectric effect : Electrons (ro electric current ) were ejected when certain metals (for example potassium rubidium caesium etc) were exposed to a beam of light . The phenomenon is called photoelectric effect.
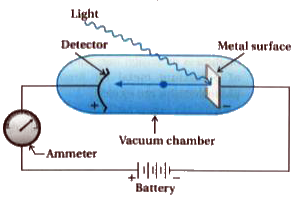
Light of a particular frequency strikes a clean metal surface inside a vacuum chamber Electrons are ejected from the metal and are counted by a detector that measures their kinetic energy.
Experimental results and assumption :
(i) The electrons are ejected from the metal surface as soon as the beam of ligth strikes the surface
Assumption : There is no time lg between the striking of light beam and the ejection of electrion from the metal surface
Assumption : (number of electron `prop` intensity
(iii) Threshold frequency : There is a characteristic minimum frequency `v_(0)` below which photoelectric effect is not observed is
Assumption : Below `v_(0)` photoelectric effect is come out with certain kinetic energy The kinetic energies of these electrons increase with the increase of frequency of the light used.
Example of threshold energy
Red light `(v= 4.3 xx 10^(4) Hz)` of any brightness (intensity ) may shine on a piece of potassium metal for hours but not photoelectrons are ejected.
A very weak yellow light `(v=5.1 ` to `5.2 xx 10^(4))`
photoelectric effect is observed.
The threshold frequency `(v_(0))` for potassium metal is `5.0 xx 10^(4) Hz`
Note : The energy content of the beam of light number of electric ejected does depend upon the brightness of light the kinetic energy of the ejected electrons does not.