(i) Principal quantum number : The stationary states for electron are numbered n = 1,2,3 These integral number are know as principal quantum numbers.
(ii) Stationary orbit radili (r ) : The radil of the stationary states are expressed as :
`r_(n) = n^(2) a_(0) `
where `a_(0) = 52.9 pm`
The radius of the first stationary (n-1) state called the Bohr.s orbit is 52.9 pm
Normally the elecrron in the hydrogen atom is found in this orbit (that is n=1)
(iii) energy of stationary state : The most important property associated with the electron is the energy of its stationary state it is given by the expression.
When the electron is free the influence of nucleus the energy is taken as zero . The electron in this situation is associated with the stationary state of principal Quantum number = in this situation electron is free from H atom and become `(H^(+))` hydrogen ion.
When the electron is attracted by the nucleus and its energy is lowered . That is the reason for the presence of negative sigh in equation and depicts its stability relative to the reference state of zero energy and n= so Energy of electrons in atom is less than the free electron.
`E_(prop) = 0`
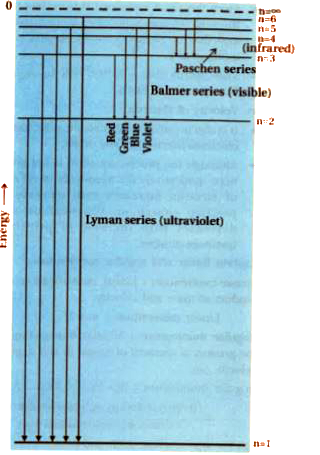
(iv) boh.s theory for isoelectronic ion of hydrogen
Bohr.s theory can also applied to the ions containing only one electron similar to that present in hydrogen atom.
The energies of the stationary states known as hydrogen like species ) are given by the expression.
`E_(n) = - 2 .18 xx 10^(-18) .(z^(2))/( n^(2)) 1`
Where z= atomic number =2,3,4 for the helium lithium beryllium respectively
From the above equations it is evident that the value of energy becomes more negative and that of radius becomes smaller with increase of Z
(v ) Velocity of electron
It is also possible to calculate teh velocities of electrons moving in these orbits ,
Although the precise equation is not given here qualitatively the magnitude of velocity positive charge on the nucleus and decreases with increase of principal quantum number
