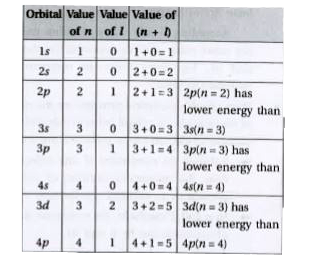
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -A (Try Your Self -1)|4 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -A (Try Your Self -2)|5 VideosSTATES OF MATTER
KUMAR PRAKASHAN|Exercise QUESTION PAPER FROM MODULE (SECTION - A)|4 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-D NCERT EXEMPLAR SOLUTION (MCQs) LONG ANSWER|25 Videos
Similar Questions
Explore conceptually related problems